Antibiotics in Raw Meat Samples: Estimation of Dietary Exposure and Risk Assessment
- PMID: 36006135
- PMCID: PMC9412356
- DOI: 10.3390/toxics10080456
Antibiotics in Raw Meat Samples: Estimation of Dietary Exposure and Risk Assessment
Abstract
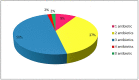
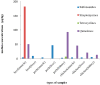
The extensive use of antibiotics in livestock farming poses increased concerns for human health as residues of these substances are present in edible tissues. The aim of this study was the determination of the levels of four groups of antibiotics (sulfonamides-SAs, tetracyclines-TCs, streptomycines-STr and quinolones-QNLs) in meat samples (muscles, livers and kidneys from beef, chicken and pork) and the estimation of the dietary exposure to antibiotics from meat consumption and the potential hazard for human health. Fifty-four samples of raw meat were randomly collected in 2018 from the Cretan market, Greece and analyzed both with an enzyme-linked immunosorbent assay (ELISA) and liquid chromatography-mass spectrometry (LC-MS). According to the results derived from the ELISA method, only 2% of the meat samples were free from antibiotics, 2% were detected with 4 antibiotics and the great majority of the samples (87%) were detected with 2 to 3 antibiotics. SAs presented the highest detection frequencies for all samples whereas TCs were not detected in any bovine sample. The highest median concentration was detected for STr in bovine muscles (182.10 μg/kg) followed by QNLs (93.36 μg/kg) in pork kidneys whereas the chicken samples had higher burdens of QNLs compared to the other meat samples. LC-MS analysis showed that oxytetracycline (OTC) was the most common antibiotic in all samples. The highest median concentration of all antibiotics was detected for doxycycline (DOX) (181.73 μg/kg in pork kidney) followed by OTC in bovine liver (74.46 μg/kg). Risk characterization was applied for each of the two methods; The hazard quotients (HQ) did not exceed 0.059 for the ELISA method and 0.113 for the LC-MS method for any group of antibiotics, whereas the total hazard indexes (HI) were 0.078 and 0.021, respectively. The results showed the presence of different groups of antibiotics in meat from the Cretan market and that the health risk to antibiotics is low. A risk assessment analysis conducted for meat consumption and corrected for the aggregated exposure revealed no risk for the consumers.
Keywords: antibiotics; meat; quinolones; risk assessment; streptomycines; sulfonamides; tetracyclines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Detection of residues of tetracycline antibiotics in pork and chicken meat: correlation between results of screening and confirmatory tests.Analyst. 1998 Dec;123(12):2737-41. doi: 10.1039/a804909b. Analyst. 1998. PMID: 10435335
-
Determination of tetracycline residues in chicken meat by liquid chromatography-tandem mass spectrometry.Food Addit Contam Part B Surveill. 2012;5(1):45-9. doi: 10.1080/19393210.2012.655782. Epub 2012 Mar 1. Food Addit Contam Part B Surveill. 2012. PMID: 24779694
-
Determination of residues of pesticides, anabolic steroids, antibiotics, and antibacterial compounds in meat products in Oman by liquid chromatography/mass spectrometry and enzyme-linked immunosorbent assay.Vet World. 2021 Mar;14(3):709-720. doi: 10.14202/vetworld.2021.709-720. Epub 2021 Mar 22. Vet World. 2021. PMID: 33935417 Free PMC article.
-
Antibiotic residue monitoring results for pork, chicken, and beef samples in Vietnam in 2012-2013.J Agric Food Chem. 2015 Jun 3;63(21):5141-5. doi: 10.1021/jf505254y. Epub 2015 Jan 28. J Agric Food Chem. 2015. PMID: 25601049
-
Occurrence and distribution of sulfonamides, tetracyclines, quinolones, macrolides, and nitrofurans in livestock manure and amended soils of Northern China.Environ Sci Pollut Res Int. 2015 Mar;22(6):4545-54. doi: 10.1007/s11356-014-3632-y. Epub 2014 Oct 16. Environ Sci Pollut Res Int. 2015. PMID: 25318415
Cited by
-
Silver Nanoparticle-Embedded Hydrogels for Electrochemical Sensing of Sulfamethoxazole Residues in Meat.Molecules. 2024 Mar 12;29(6):1256. doi: 10.3390/molecules29061256. Molecules. 2024. PMID: 38542893 Free PMC article.
-
Prototype for rapid test devices to detect residues of sulfonamides in chicken carcasses from traditional breeders in Surabaya, Indonesia.Vet World. 2023 Jun;16(6):1252-1259. doi: 10.14202/vetworld.2023.1252-1259. Epub 2023 Jun 8. Vet World. 2023. PMID: 37577197 Free PMC article.
-
Multiresidues Multiclass Analytical Methods for Determination of Antibiotics in Animal Origin Food: A Critical Analysis.Antibiotics (Basel). 2023 Jan 18;12(2):202. doi: 10.3390/antibiotics12020202. Antibiotics (Basel). 2023. PMID: 36830113 Free PMC article. Review.
References
-
- Diaz-Sanchez S., Moscoso S., Solís de los Santos F., Andino A., Hanning I. Antibiotic use in poultry; A driving force for organic poultry production. Food Prot. Trends. 2015;35:440–447.
-
- Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on Additives for Use in Animal Nutrition. [(accessed on 29 November 2018)]. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32003R1831.
-
- Directive 2004/28/EC of the European Parliament and of the Council of 31 March 2004 Amending Directive 2001/82/EC on the Community Code Relating to Veterinary Medicinal Products. [(accessed on 29 November 2018)]. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32004L0028.
-
- Kuriyama T., Karasawa T., Williams D.W. Chapter Thirteen-Antimicrobial Chemotherapy: Significance to Healthcare. In: Steven L., David P., Williams W., Randle J., Cooper T., editors. Biofilms in Infection Prevention and Control. Academic Press; Cambridge, MA, USA: 2014. pp. 209–244.
LinkOut - more resources
Full Text Sources