Recognition of Heavy Metals by Using Resorcin[4]arenes Soluble in Water
- PMID: 36006140
- PMCID: PMC9415269
- DOI: 10.3390/toxics10080461
Recognition of Heavy Metals by Using Resorcin[4]arenes Soluble in Water
Abstract
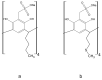
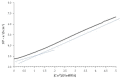
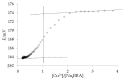
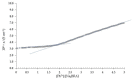
The complexing properties of two water-soluble resorcin[4]arenes (tetrasodium 5,11,17,23-tetrakissulfonatemethylen 2,8,14,20-tetra(butyl)resorcin[4]arene, Na4BRA, and tetrasodium 5,11,17,23-tetrakissulfonatemethylen-2,8,14,20-tetra(2-(methylthio)ethyl)resorcin[4]arene, Na4SRA) with polluting heavy metals such as Cu2+, Pb2+, Cd2+ and Hg2+ were studied by conductivity, and the findings were confirmed by using other techniques to try to apply this knowledge to removing them. The results indicate that Na4BRA is able to complex Cu2+ in a 1:1 ratio and Pb2+ in a 1:2 ratio, while Na4SRA complexes Hg2+ in a 1:1 ratio. On the contrary, no indications have been observed that either of the resorcin[4]arenes studied complexes the Cd2+ ions. The results suggest that the bonds established between the sulfur atoms located at the lower edge of the SRA4- and the solvent hydrogens could prevent the entry of the guest into the host cavity. However, in the case of Hg2+ ions, the entry is favoured by the interactions between the sulfur donor atoms present on the lower edge of Na4SRA and the Hg2+ ions. Therefore, it can be said that Na4BRA is selective for Cu2+ and Pb2+ ions and Na4SRA is selective for Hg2+ ions.
Keywords: heavy metals; inorganic pollutants; recognition; resorcin[4]arenes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Rahman Z., Jagadheeswari , Mohan A., Priya S., Tharini , Selvendran Electrokinetic remediation: An innovation for heavy metal contamination in the soil environment. Mater. Today Proc. 2021;37:2730–2734. doi: 10.1016/j.matpr.2020.08.541. - DOI
LinkOut - more resources
Full Text Sources

