In Vitro Effects of Fungal Phytotoxins on Cancer Cell Viability: First Insight into Structure Activity Relationship of a Potent Metabolite of Cochliobolus australiensis Radicinin
- PMID: 36006179
- PMCID: PMC9415302
- DOI: 10.3390/toxins14080517
In Vitro Effects of Fungal Phytotoxins on Cancer Cell Viability: First Insight into Structure Activity Relationship of a Potent Metabolite of Cochliobolus australiensis Radicinin
Abstract
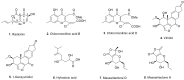
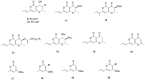
Natural compounds have always represented an important source for new drugs. Although fungi represent one such viable source, to date, no fungal metabolite has been marketed as an anticancer drug. Based on our work with phytotoxins as potential chemical scaffolds and our recent findings involving three phytopathogenic fungi, i.e., Cochliobolus australiensis, Kalmusia variispora and Hymenoscyphus fraxineus, herein, we evaluate the in vitro anti-cancer activity of the metabolites of these fungi by MTT assays on three cancer cell models harboring various resistance levels to chemotherapeutic drugs. Radicinin, a phytotoxic dihydropyranopyran-4,5-dione produced by Cochliobolus australiensis, with great potential for the biocontrol of the invasive weed buffelgrass (Cenchrus ciliaris), showed significant anticancer activity in the micromolar range. Furthermore, a SAR study was carried out using radicinin, some natural analogues and hemisynthetic derivatives prepared by synthetic methods developed as part of work aimed at the potential application of these molecules as bioherbicides. This investigation opens new avenues for the design and synthesis of novel radicinin analogues as potential anticancer agents.
Keywords: Cochliobolus australiensis; SAR study; anticancer; fungi; natural and hemisynthetic derivatives; phytotoxins; radicinin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Phytotoxic Activity and Structure-Activity Relationships of Radicinin Derivatives against the Invasive Weed Buffelgrass (Cenchrus ciliaris).Molecules. 2019 Jul 31;24(15):2793. doi: 10.3390/molecules24152793. Molecules. 2019. PMID: 31370299 Free PMC article.
-
Cochliotoxin, a Dihydropyranopyran-4,5-dione, and Its Analogues Produced by Cochliobolus australiensis Display Phytotoxic Activity against Buffelgrass (Cenchrus ciliaris).J Nat Prod. 2017 May 26;80(5):1241-1247. doi: 10.1021/acs.jnatprod.6b00696. Epub 2017 Apr 19. J Nat Prod. 2017. PMID: 28422495
-
Effect of cultural conditions on the production of radicinin, a specific fungal phytotoxin for buffelgrass (Cenchrus ciliaris) biocontrol, by different Cochlioboulus australiensis strains.Nat Prod Res. 2021 Jan;35(1):99-107. doi: 10.1080/14786419.2019.1614583. Epub 2019 Jun 5. Nat Prod Res. 2021. PMID: 31163992
-
Fungal phytotoxins with potential herbicidal activity: chemical and biological characterization.Nat Prod Rep. 2015 Dec 19;32(12):1629-53. doi: 10.1039/c5np00081e. Epub 2015 Oct 7. Nat Prod Rep. 2015. PMID: 26443032 Review.
-
Fungal Phytotoxins in Sustainable Weed Management.Curr Med Chem. 2018;25(2):268-286. doi: 10.2174/0929867324666170426152331. Curr Med Chem. 2018. PMID: 28462700 Review.
Cited by
-
Semisynthetic derivatives of massarilactone D with cytotoxic and nematicidal activities.Beilstein J Org Chem. 2025 Mar 17;21:607-615. doi: 10.3762/bjoc.21.48. eCollection 2025. Beilstein J Org Chem. 2025. PMID: 40130181 Free PMC article.
-
An Overview of α-Pyrones as Phytotoxins Produced by Plant Pathogen Fungi.Molecules. 2025 Jun 30;30(13):2813. doi: 10.3390/molecules30132813. Molecules. 2025. PMID: 40649327 Free PMC article. Review.
-
Advances on anticancer fungal metabolites: sources, chemical and biological activities in the last decade (2012-2023).Nat Prod Bioprospect. 2024 May 14;14(1):31. doi: 10.1007/s13659-024-00452-0. Nat Prod Bioprospect. 2024. PMID: 38743184 Free PMC article.
-
(±)-3-Deoxyradicinin Induces Stomata Opening and Chloroplast Oxidative Stress in Tomato (Solanum lycopersicum L.).Int J Mol Sci. 2023 May 9;24(10):8467. doi: 10.3390/ijms24108467. Int J Mol Sci. 2023. PMID: 37239812 Free PMC article.
-
Preliminary Studies on the In Vitro Interactions Between the Secondary Metabolites Produced by Esca-Associated Fungi and Enological Saccharomyces cerevisiae Strains.Plants (Basel). 2022 Aug 31;11(17):2277. doi: 10.3390/plants11172277. Plants (Basel). 2022. PMID: 36079659 Free PMC article.
References
-
- Hyde K.D., Jianchu X., Rapior S., Jeewon R., Lumyong S., Nieg A.G.T., Pranami D., Abeywickrama P.D., ·Aluthmuhandiram J.V.S., Brahamanage R.S., et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Diver. 2019;97:1–136. doi: 10.1007/s13225-019-00430-9. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

