Equatorial Spitting Cobra (Naja sumatrana) from Malaysia (Negeri Sembilan and Penang), Southern Thailand, and Sumatra: Comparative Venom Proteomics, Immunoreactivity and Cross-Neutralization by Antivenom
- PMID: 36006183
- PMCID: PMC9414237
- DOI: 10.3390/toxins14080522
Equatorial Spitting Cobra (Naja sumatrana) from Malaysia (Negeri Sembilan and Penang), Southern Thailand, and Sumatra: Comparative Venom Proteomics, Immunoreactivity and Cross-Neutralization by Antivenom
Abstract
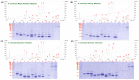
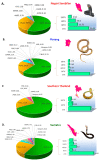
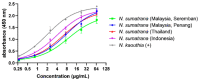
The Equatorial Spitting Cobra (Naja sumatrana) is a medically important venomous snake species in Southeast Asia. Its wide geographical distribution implies potential intra-specific venom variation, while there is no species-specific antivenom available to treat its envenoming. Applying a protein-decomplexing proteomic approach, the study showed that three-finger toxins (3FTX), followed by phospholipases A2 (PLA2), were the major proteins well-conserved across N. sumatrana venoms of different locales. Variations were noted in the subtypes and relative abundances of venom proteins. Of note, alpha-neurotoxins (belonging to 3FTX) are the least in the Penang specimen (Ns-PG, 5.41% of total venom proteins), compared with geographical specimens from Negeri Sembilan (Ns-NS, 14.84%), southern Thailand (Ns-TH, 16.05%) and Sumatra (Ns-SU, 10.81%). The alpha-neurotoxin abundance, in general, correlates with the venom's lethal potency. The Thai Naja kaouthia Monovalent Antivenom (NkMAV) was found to be immunoreactive toward the N. sumatrana venoms and is capable of cross-neutralizing N. sumatrana venom lethality to varying degrees (potency = 0.49-0.92 mg/mL, interpreted as the amount of venom completely neutralized per milliliter of antivenom). The potency was lowest against NS-SU venom, implying variable antigenicity of its lethal alpha-neurotoxins. Together, the findings suggest the para-specific and geographical utility of NkMAV as treatment for N. sumatrana envenoming in Southeast Asia.
Keywords: Naja kaouthia monovalent antivenom (NkMAV); antivenom potency; monocled cobra antivenom; snakebite envenoming; venom variation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kasturiratne A., Wickremasinghe A.R., de Silva N., Gunawardena N.K., Pathmeswaran A., Premaratna R., Savioli L., Lalloo D.G., de Silva H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:e218. doi: 10.1371/journal.pmed.0050218. - DOI - PMC - PubMed
-
- WHO . Guidelines for the Management of Snakebites. WHO Regional Office for Southeast Asia; New Delhi, India: 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

