Diversity of Phospholipases A2 from Bothrops atrox Snake Venom: Adaptive Advantages for Snakes Compromising Treatments for Snakebite Patients
- PMID: 36006204
- PMCID: PMC9414272
- DOI: 10.3390/toxins14080543
Diversity of Phospholipases A2 from Bothrops atrox Snake Venom: Adaptive Advantages for Snakes Compromising Treatments for Snakebite Patients
Abstract
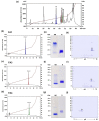
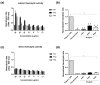
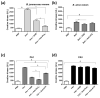
The evolution of snake venoms resulted in multigene toxin families that code for structurally similar isoforms eventually harboring distinct functions. PLA2s are dominant toxins in viper venoms, and little is known about the impact of their diversity on human envenomings and neutralization by antivenoms. Here, we show the isolation of three distinct PLA2s from B. atrox venom. FA1 is a Lys-49 homologue, and FA3 and FA4 are catalytic Asp-49 PLA2s. FA1 and FA3 are basic myotoxic proteins, while FA4 is an acid non-myotoxic PLA2. FA3 was the most potent toxin, inducing higher levels of edema, inflammatory nociception, indirect hemolysis, and anticoagulant activity on human, rat, and chicken plasmas. FA4 presented lower anticoagulant activity, and FA1 had only a slight effect on human and rat plasmas. PLA2s presented differential reactivities with antivenoms, with an emphasis on FA3, which was not recognized or neutralized by the antivenoms used in this study. Our findings reveal the functional and antigenic diversity among PLA2s from B. atrox venom, highlighting the importance of assessing venom variability for understanding human envenomations and treatment with antivenoms, particularly evident here as the antivenom fails to recognize FA3, the most active multifunctional toxin described.
Keywords: PLA2; antivenom; diversity; snake venom; snakebites.
Conflict of interest statement
The authors declare no conflict of interest regarding the publication of this paper. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



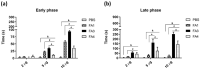


Similar articles
-
Antivenomics and in vivo preclinical efficacy of six Latin American antivenoms towards south-western Colombian Bothrops asper lineage venoms.PLoS Negl Trop Dis. 2021 Feb 1;15(2):e0009073. doi: 10.1371/journal.pntd.0009073. eCollection 2021 Feb. PLoS Negl Trop Dis. 2021. PMID: 33524033 Free PMC article.
-
Development of Nanobodies Against Hemorrhagic and Myotoxic Components of Bothrops atrox Snake Venom.Front Immunol. 2020 May 7;11:655. doi: 10.3389/fimmu.2020.00655. eCollection 2020. Front Immunol. 2020. PMID: 32457735 Free PMC article.
-
Pan-American Lancehead Pit-Vipers: Coagulotoxic Venom Effects and Antivenom Neutralisation of Bothrops asper and B. atrox Geographical Variants.Toxins (Basel). 2021 Jan 22;13(2):78. doi: 10.3390/toxins13020078. Toxins (Basel). 2021. PMID: 33499001 Free PMC article.
-
Snakebite envenoming in French Guiana: Assessment of the preclinical efficacy against the venom of Bothrops atrox of two polyspecific antivenoms.Toxicon. 2020 Jan 15;173:1-4. doi: 10.1016/j.toxicon.2019.11.001. Epub 2019 Nov 11. Toxicon. 2020. PMID: 31726079 Review.
-
Inflammatory effects of snake venom myotoxic phospholipases A2.Toxicon. 2003 Dec 15;42(8):947-62. doi: 10.1016/j.toxicon.2003.11.006. Toxicon. 2003. PMID: 15019493 Review.
Cited by
-
Enhancing the Efficacy of Melanoma Treatment: The In Vitro Chemosensitising Impact of Vipera ammodytes Venom on Human Melanoma Cell Lines.Toxins (Basel). 2025 Mar 21;17(4):152. doi: 10.3390/toxins17040152. Toxins (Basel). 2025. PMID: 40278650 Free PMC article.
-
Biochemical characterization of the venom of the Bolivian endemic pit viper Bothrops sanctaecrucis.Toxicon X. 2025 Feb 18;25:100216. doi: 10.1016/j.toxcx.2025.100216. eCollection 2025 Mar. Toxicon X. 2025. PMID: 40104440 Free PMC article.
-
Qualitative Profiling of Venom Toxins in the Venoms of Several Bothrops Species Using High-Throughput Venomics and Coagulation Bioassaying.Toxins (Basel). 2024 Jul 1;16(7):300. doi: 10.3390/toxins16070300. Toxins (Basel). 2024. PMID: 39057940 Free PMC article.
References
-
- Amazonas D.R., Portes-Junior J.A., Nishiyama M.Y., Jr., Nicolau C.A., Chalkidis H.M., Mourão R.H.V., Grazziotin F.G., Rokyta D.R., Gibbs H.L., Valente R.H., et al. Molecular mechanisms underlying intraspecific variation in snake venom. J. Proteom. 2018;181:60–72. doi: 10.1016/j.jprot.2018.03.032. - DOI - PubMed
-
- Zancolli G., Calvete J.J., Cardwell M.D., Greene H.W., Hayes W.K., Hegarty M.J., Herrmann H.W., Holycross A.T., Lannutti D.I., Mulley J.F., et al. When one phenotype is not enough: Divergent evolutionary trajectories govern venom variation in a widespread rattlesnake species. Proc. Biol. Sci. 2019;286:20182735. doi: 10.1098/rspb.2018.2735. - DOI - PMC - PubMed
-
- Sousa L.F., Portes-Junior J.A., Nicolau C.A., Bernardoni J.L., Nishiyama M.Y., Amazonas D.R., Freitas-de-Sousa L.A., Mourão R.H., Chalkidis H.M., Valente R.H., et al. Functional proteomic analyses of Bothrops atrox venom reveals phenotypes associated with habitat variation in the Amazon. J. Proteom. 2017;159:32–46. doi: 10.1016/j.jprot.2017.03.003. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

