Effect of Botulinum Toxin Injection on EMG Activity and Bite Force in Masticatory Muscle Disorder: A Randomized Clinical Trial
- PMID: 36006207
- PMCID: PMC9416064
- DOI: 10.3390/toxins14080545
Effect of Botulinum Toxin Injection on EMG Activity and Bite Force in Masticatory Muscle Disorder: A Randomized Clinical Trial
Abstract
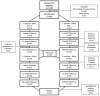
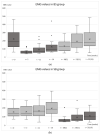
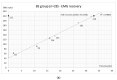
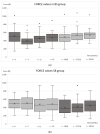
Botulinum toxin type A (BoNT-A) is increasingly used in treating masticatory muscle pain disorder; however, safe doses and reinjection intervals still need to be established. The purpose of this randomized clinical trial was to evaluate the degree and duration of the impairment of masticatory muscle performance. Fifty-seven subjects were randomly divided into two groups: one of which received BoNT-A first (n = 28) while the other received saline first (n = 29), with the cross-over being in week 16, and a total follow-up period of 32 weeks. A total dose of 50 U of BoNT-A was injected in the masseter and temporal muscles bilaterally. Electromyographic (EMG) activity and bite forces were assessed. A significant reduction in EMG activity was observed up to week 18 (p ≤ 001), with total recovery at week 33. A significant reduction in maximum bite force was observed up to week 11 (p ≤ 005), with total recovery at week 25. In conclusion, when treating masticatory muscle pain disorder with 50 U of BoNT-A, a reinjection interval of 33 weeks can be considered safe since the recovery of muscle function occurs by that time.
Keywords: botulinum toxin type A; chronic pain; masticatory muscle disorder; myofascial pain; temporomandibular disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Repeated botulinum treatment of rabbit masseter causes cumulative tissue damage.Arch Oral Biol. 2022 Sep;141:105480. doi: 10.1016/j.archoralbio.2022.105480. Epub 2022 Jun 11. Arch Oral Biol. 2022. PMID: 35724521
-
Electromyography and bite force studies of muscular function and dysfunction in masticatory muscles.Swed Dent J Suppl. 1986;37:1-64. Swed Dent J Suppl. 1986. PMID: 3465056 Clinical Trial.
-
Bilateral treatment of the masseter with botulinum toxin: Consequences for mastication, muscle force and the mandibular condyle.J Oral Rehabil. 2023 Sep;50(9):775-781. doi: 10.1111/joor.13495. Epub 2023 May 24. J Oral Rehabil. 2023. PMID: 37178264
-
Systematic review of the histological and functional effects of botulinum toxin A on masticatory muscles: Consideration in dentofacial orthopedics and orthognathic surgery.Ann Anat. 2024 Oct;256:152302. doi: 10.1016/j.aanat.2024.152302. Epub 2024 Jul 20. Ann Anat. 2024. PMID: 39038690
-
Mandibular Bone Loss after Masticatory Muscles Intervention with Botulinum Toxin: An Approach from Basic Research to Clinical Findings.Toxins (Basel). 2019 Feb 1;11(2):84. doi: 10.3390/toxins11020084. Toxins (Basel). 2019. PMID: 30717172 Free PMC article. Review.
Cited by
-
Surface Electromyography in Dentistry-Past, Present and Future.J Clin Med. 2024 Feb 26;13(5):1328. doi: 10.3390/jcm13051328. J Clin Med. 2024. PMID: 38592144 Free PMC article. Review.
-
Analysis of the Use of Sample Size and Effect Size Calculations in a Temporomandibular Disorders Randomised Controlled Trial-Short Narrative Review.J Pers Med. 2024 Jun 19;14(6):655. doi: 10.3390/jpm14060655. J Pers Med. 2024. PMID: 38929876 Free PMC article. Review.
-
Botulinum Toxin for Bruxism: An Overview.Toxins (Basel). 2025 May 16;17(5):249. doi: 10.3390/toxins17050249. Toxins (Basel). 2025. PMID: 40423331 Free PMC article.
-
A Comparative Analysis of Botulinum Toxin Use Versus Other Therapies for Temporomandibular Disorders: A Systematic Review.Cureus. 2024 Sep 28;16(9):e70389. doi: 10.7759/cureus.70389. eCollection 2024 Sep. Cureus. 2024. PMID: 39469401 Free PMC article. Review.
-
Incobotulinumtoxin A and Yoga-like Isometric Exercise in Adolescent Idiopathic Lumbar Scoliosis-A Randomized Pilot Study.Muscles. 2024 Feb 1;3(1):28-39. doi: 10.3390/muscles3010004. Muscles. 2024. PMID: 40757545 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

