The Lack of Systemic and Subclinical Side Effects of Botulinum Neurotoxin Type-A in Patients Affected by Post-Stroke Spasticity: A Longitudinal Cohort Study
- PMID: 36006227
- PMCID: PMC9414297
- DOI: 10.3390/toxins14080564
The Lack of Systemic and Subclinical Side Effects of Botulinum Neurotoxin Type-A in Patients Affected by Post-Stroke Spasticity: A Longitudinal Cohort Study
Abstract
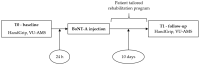
Botulinum Neurotoxin type-A (BoNT-A) is the treatment of choice for focal post-stroke spasticity (PSS). Due to its mechanism of action and the administration method, some authors raised concern about its possible systemic diffusion leading to contralateral muscle weakness and autonomic nervous system (ANS) alterations. Stroke itself is a cause of motor disability and ANS impairment; therefore, it is mandatory to prevent any source of additional loss of strength and adjunctive ANS disturbance. We enrolled 15 hemiparetic stroke survivors affected by PSS already addressed to BoNT-A treatment. Contralateral handgrip strength and ANS parameters, such as heart rate variability, impedance cardiography values, and respiratory sinus arrythmia, were measured 24 h before (T0) and 10 days after (T1) the ultrasound (US)-guided BoNT-A injection. At T1, neither strength loss nor modification of the basal ANS patterns were found. These findings support recent literature about the safety profile of BoNT-A, endorsing the importance of the US guide for a precise targeting and the sparing of "critical" structures as vessels and nerves.
Keywords: autonomic nervous system; botulinum toxin; heart rate; muscle spasticity; rehabilitation; stroke.
Conflict of interest statement
The authors declare no conflict of interest.
Similar articles
-
Contralateral weakness following botulinum toxin for poststroke spasticity.Muscle Nerve. 2012 Sep;46(3):443-8. doi: 10.1002/mus.23492. Muscle Nerve. 2012. PMID: 22907238
-
Botulinum toxin injection for hypertonicity of the upper extremity within 12 weeks after stroke: a randomized controlled trial.Neurorehabil Neural Repair. 2012 Sep;26(7):812-21. doi: 10.1177/1545968311430824. Epub 2012 Feb 27. Neurorehabil Neural Repair. 2012. PMID: 22371239 Clinical Trial.
-
Safety Profile of High-Dose Botulinum Toxin Type A in Post-Stroke Spasticity Treatment.Clin Drug Investig. 2018 Nov;38(11):991-1000. doi: 10.1007/s40261-018-0701-x. Clin Drug Investig. 2018. PMID: 30209743 Review.
-
The Effects of Botulinum Toxin Injections on Spasticity and Motor Performance in Chronic Stroke with Spastic Hemiplegia.Toxins (Basel). 2020 Jul 31;12(8):492. doi: 10.3390/toxins12080492. Toxins (Basel). 2020. PMID: 32751970 Free PMC article. Clinical Trial.
-
Botulinum toxin as early intervention for spasticity after stroke or non-progressive brain lesion: A meta-analysis.J Neurol Sci. 2016 Dec 15;371:6-14. doi: 10.1016/j.jns.2016.10.005. Epub 2016 Oct 11. J Neurol Sci. 2016. PMID: 27871449 Review.
Cited by
-
Delphi Consensus on the Management of Spanish Patients with Post-Stroke Hemiplegic Shoulder Pain Treated with Botulinum Toxin A: Result Study.Toxins (Basel). 2025 Jan 16;17(1):40. doi: 10.3390/toxins17010040. Toxins (Basel). 2025. PMID: 39852993 Free PMC article.
-
Self-Administered Botulinum Injection Causing Rare Masquerade of Systemic Botulism.Cureus. 2024 Jul 20;16(7):e64976. doi: 10.7759/cureus.64976. eCollection 2024 Jul. Cureus. 2024. PMID: 39161519 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical