Physiological Response of Atlantic Salmon (Salmo salar) to Long-Term Exposure to an Anesthetic Obtained from Heterosigma akashiwo
- PMID: 36006237
- PMCID: PMC9416519
- DOI: 10.3390/toxins14080575
Physiological Response of Atlantic Salmon (Salmo salar) to Long-Term Exposure to an Anesthetic Obtained from Heterosigma akashiwo
Abstract
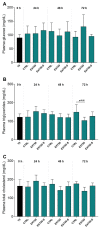
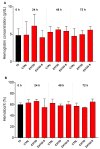
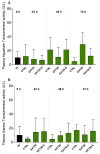
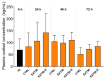
Despite the invaluable role of anesthetics as a tool for ensuring animal welfare in stressful situations, there is currently a lack of anesthetic drugs that meet the requirements of intensive aquaculture. In response to the growing interest in anesthetic substances of natural origin, this study evaluated the physiological and health impact of an anesthetic based on an extract of the microalga Heterosigma akashiwo on juvenile salmon (Salmo salar) exposed for a period of 72 h. To simulate a condition closer to reality where fish are subjected to stimuli (e.g., transport), the animals were exposed to 50 mg L-1 of algal extract and to physical stress. Functional, physiological, and histological parameters were evaluated in blood and tissues at different sampling periods (0, 24, and 72 h). There was no mortality and the induction and recovery times observed were within the established criteria for anesthetic efficacy. The anesthetic extract did not induce any side effects, such as stress or metabolic damage, indicating that this extract is a viable option for supporting fish welfare during deleterious events. This study provides information to support that the anesthetic extract tested, derived from H. akashiwo, is a promising candidate drug for operations requiring sedation (e.g., Salmonid transport).
Keywords: Atlantic salmon (Salmo salar); natural anesthetic; physiological response; welfare practices.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sadoul B., Vijayan M.M. Fish Physiology. Volume 35. Elsevier Inc.; Amsterdam, The Netherlands: 2016. Stress and Growth; pp. 167–205.
-
- Priborsky J., Velisek J. A Review of Three Commonly Used Fish Anesthetics. Rev. Fish. Sci. Aquac. 2018;26:417–442. doi: 10.1080/23308249.2018.1442812. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

