Recombinant Ehrlichia canis GP19 Protein as a Promising Vaccine Prototype Providing a Protective Immune Response in a Mouse Model
- PMID: 36006302
- PMCID: PMC9414908
- DOI: 10.3390/vetsci9080386
Recombinant Ehrlichia canis GP19 Protein as a Promising Vaccine Prototype Providing a Protective Immune Response in a Mouse Model
Abstract
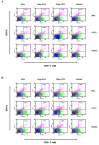
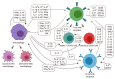
The intracellular bacterium Ehrlichia canis is the causative pathogen of canine monocytic ehrlichiosis (CME) in dogs. Despite its veterinary and medical importance, there is currently no available vaccine against this pathogen. In this study, the recombinant GP19 (rGP19) was produced and used as a recombinant vaccine prototype in a mouse model against experimental E. canis infection. The efficacy of the rGP19 vaccine prototype in the part of stimulating B and T cell responses and conferring protection in mice later challenged with E. canis pathogen were evaluated. The rGP19-specific antibody response was evaluated by ELISA after E. canis challenge exposure (on days 0, 7, and 14 post-challenge), and demonstrated significantly higher mean antibody levels in rGP19-immunized mice compared with adjuvant-immunized and naive mice. Significantly lower ehrlichial loads in blood, liver, and spleen DNA samples were detected in the immunized mice with rGP19 by qPCR. The up-regulation of IFNG and IL1 mRNA expression were observed in mice immunized with rGP19. In addition, this study detected IFN-γ-producing memory CD4+ T cells in the rGP19-immunized mice and later infected with E. canis on day 14 post-infection period using flow cytometry. The present study provided a piece of evidence that rGP19 may eliminate E. canis by manipulating Th1 and B cell roles and demonstrated a promising strategy in vaccine development against E. canis infection in the definitive host for further study.
Keywords: CME; Ehrlichia canis; GP19; mice; recombinant protein; vaccine prototype.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Ehrlichia canis Vaccine Development: Challenges and Advances.Vet Sci. 2024 Dec 5;11(12):624. doi: 10.3390/vetsci11120624. Vet Sci. 2024. PMID: 39728964 Free PMC article. Review.
-
Recombinant gp19 as a potential antigen for detecting anti-Ehrlichia canis antibodies in dog sera.Rev Bras Parasitol Vet. 2015 Jul-Sep;24(3):290-7. doi: 10.1590/S1984-29612015055. Epub 2015 Aug 14. Rev Bras Parasitol Vet. 2015. PMID: 26291145
-
Enzyme-linked immunosorbent assay with conserved immunoreactive glycoproteins gp36 and gp19 has enhanced sensitivity and provides species-specific immunodiagnosis of Ehrlichia canis infection.Clin Vaccine Immunol. 2007 Feb;14(2):123-8. doi: 10.1128/CVI.00361-06. Epub 2006 Dec 6. Clin Vaccine Immunol. 2007. PMID: 17151186 Free PMC article.
-
Immunization with Ehrlichia P28 outer membrane proteins confers protection in a mouse model of ehrlichiosis.Clin Vaccine Immunol. 2011 Dec;18(12):2018-25. doi: 10.1128/CVI.05292-11. Epub 2011 Oct 26. Clin Vaccine Immunol. 2011. PMID: 22030371 Free PMC article.
-
Significance of serological testing for ehrlichial diseases in dogs with special emphasis on the diagnosis of canine monocytic ehrlichiosis caused by Ehrlichia canis.Vet Parasitol. 2001 Feb;95(1):1-15. doi: 10.1016/s0304-4017(00)00407-6. Vet Parasitol. 2001. PMID: 11163693 Review.
Cited by
-
Antibody reactive immunomes of Ehrlichia chaffeensis and E. canis are diverse and defined by conformational antigenic determinants.Front Cell Infect Microbiol. 2024 Jan 9;13:1321291. doi: 10.3389/fcimb.2023.1321291. eCollection 2023. Front Cell Infect Microbiol. 2024. PMID: 38264730 Free PMC article.
-
Ehrlichia canis Vaccine Development: Challenges and Advances.Vet Sci. 2024 Dec 5;11(12):624. doi: 10.3390/vetsci11120624. Vet Sci. 2024. PMID: 39728964 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

