Sphingolipidomics of Bovine Pink Eye: A Pilot Study
- PMID: 36006303
- PMCID: PMC9414827
- DOI: 10.3390/vetsci9080388
Sphingolipidomics of Bovine Pink Eye: A Pilot Study
Abstract
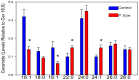
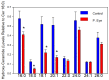
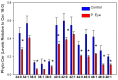
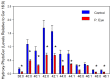
Sphingolipids are essential structural components of tear film that protect the surface of the eye from dehydration. A detailed analysis of the effects of pink eye infections on the sphingolipidome in cattle has not previously been undertaken. We recently published a new assay utilizing high-resolution mass spectrometric monitoring of the chloride adducts of sphingolipids that provides enhanced sensitivity and specificity. Utilizing this assay, we monitored decreases in the levels of tear film ceramides with short-chain fatty acids, hydroxy-ceramides, phytoceramides, and hydroxy-phytoceramides. Dihydroceramide levels were unaltered and increased levels of ceramides with long-chain fatty acids (24:0 and 24:1) were monitored in cattle with pink eye. The data from this pilot study (n = 8 controls and 8 pink eye) demonstrate a major disruption of the lipid tear film layer in pink eye disease, that can result in severe eye irritation and damage.
Keywords: Infectious Bovine Keratoconjunctivosous (IBK); bovine pink eye; sphingolipids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Tear Film Amphiphilic and Anti-Inflammatory Lipids in Bovine Pink Eye.Metabolites. 2018 Nov 21;8(4):81. doi: 10.3390/metabo8040081. Metabolites. 2018. PMID: 30469369 Free PMC article.
-
Phytoceramides from the Marine Sponge Monanchora clathrata: Structural Analysis and Cytoprotective Effects.Biomolecules. 2023 Apr 14;13(4):677. doi: 10.3390/biom13040677. Biomolecules. 2023. PMID: 37189423 Free PMC article.
-
Sphingolipidomics: An Important Mechanistic Tool for Studying Fungal Pathogens.Front Microbiol. 2016 Apr 14;7:501. doi: 10.3389/fmicb.2016.00501. eCollection 2016. Front Microbiol. 2016. PMID: 27148190 Free PMC article. Review.
-
Structure-specific, quantitative methods for analysis of sphingolipids by liquid chromatography-tandem mass spectrometry: "inside-out" sphingolipidomics.Methods Enzymol. 2007;432:83-115. doi: 10.1016/S0076-6879(07)32004-1. Methods Enzymol. 2007. PMID: 17954214
-
Component Causes of Infectious Bovine Keratoconjunctivitis-Non-Moraxella Organisms in the Epidemiology of Infectious Bovine Keratoconjunctivitis.Vet Clin North Am Food Anim Pract. 2021 Jul;37(2):295-308. doi: 10.1016/j.cvfa.2021.03.005. Vet Clin North Am Food Anim Pract. 2021. PMID: 34049660 Review.
References
LinkOut - more resources
Full Text Sources

