Comparison between Some Phenotypic and Genotypic Methods for Assessment of Antimicrobial Resistance Trend of Bovine Mastitis Staphylococcus aureus Isolates from Bulgaria
- PMID: 36006316
- PMCID: PMC9416698
- DOI: 10.3390/vetsci9080401
Comparison between Some Phenotypic and Genotypic Methods for Assessment of Antimicrobial Resistance Trend of Bovine Mastitis Staphylococcus aureus Isolates from Bulgaria
Abstract
The aim of this study was to assess the resistance of bovine mastitis S. aureus isolates from farms in Bulgaria to different classes of chemotherapeutic drugs by comparison of some phenotypic and genotypic methods by means of Cohen's kappa statistics. The study comprised 546 milk samples from subclinical and clinical mastitis at 14 farms from 9 districts in the country. A total of 92 Staphylococcus aureus strains were isolated from tested samples and identified by nuc PCR. The results demonstrated high levels of resistance to sulfadimethoxine (87%), followed by resistance to penicillin (33.7%), erythromycin (13%), streptomycin (8.7%), tetracycline (6.5%) and gentamicin (1.1%). The comparison of both phenotypic tests with respect to 9 antimicrobials revealed strong agreement with kappa coefficient 0.836. An almost complete agreement was evidenced between phenotypic resistance to penicillin and blaZ gene presence, to methicillin with mecA gene, to tetracycline with tet genes, but the agreement between erythromycin resistance and erm genes presence was moderate. This study was the first to demonstrate discrepancy between the behaviour to cefoxitin in the disk diffusion test and oxacillin in the MIC test for an isolate shown to carry the mecA gene in the subsequent genetic analysis. Considering the detected discrepancies for some of isolates, an integral evaluation through phenotypic and molecular methods for monitoring of antimicrobial resistance of Staphylococcus aureus is recommended.
Keywords: Staphylococcus aureus; antimicrobial resistance; cows; mastitis; resistance genes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
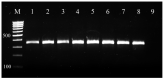

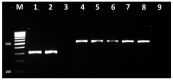


References
-
- Taylor T.A., Unakal C.G. Staphylococcus Aureus. [(accessed on 14 February 2022)];StatPearls. Treasure Island (FL): StatPearls. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441868/
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

