Make it double: identification and characterization of a Tandem-Hirudin from the Asian medicinal leech Hirudinaria manillensis
- PMID: 36006484
- PMCID: PMC9464118
- DOI: 10.1007/s00436-022-07634-0
Make it double: identification and characterization of a Tandem-Hirudin from the Asian medicinal leech Hirudinaria manillensis
Abstract
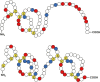
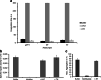
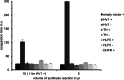
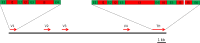
Haematophagous leeches express a broad variety of secretory proteins in their salivary glands, among them are hirudins and hirudin-like factors. Here, we describe the identification, molecular and initial functional characterization of Tandem-Hirudin (TH), a novel salivary gland derived factor identified in the Asian medicinal leech, Hirudinaria manillensis. In contrast to the typical structure of hirudins, TH comprises two globular domains arranged in a tandem-like orientation and lacks the elongated C-terminal tail. Similar structures of thrombin inhibitors have so far been identified only in kissing bugs and ticks. Expression of TH was performed in both cell-based and cell-free bacterial systems. A subsequent functional characterization revealed no evidence for a thrombin-inhibitory potency of TH.
Keywords: Blood coagulation; Hirudin; Hirudin-like factors; Hirudinaria manillensis; Medicinal leeches; Tandem-Hirudin.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Hirudins of the Asian medicinal leech, Hirudinaria manillensis: same same, but different.Parasitol Res. 2019 Jul;118(7):2223-2233. doi: 10.1007/s00436-019-06365-z. Epub 2019 Jun 11. Parasitol Res. 2019. PMID: 31187225
-
Short tail stories: the hirudin-like factors HLF6 and HLF7 of the Asian medicinal leech, Hirudinaria manillensis.Parasitol Res. 2021 Nov;120(11):3761-3769. doi: 10.1007/s00436-021-07316-3. Epub 2021 Oct 2. Parasitol Res. 2021. PMID: 34599360 Free PMC article.
-
The complete amino acid sequence of a hirudin variant from the leech Hirudinaria manillensis.J Protein Chem. 1993 Jun;12(3):365-70. doi: 10.1007/BF01028198. J Protein Chem. 1993. PMID: 8397794
-
Thrombin inhibitors of bloodsucking animals.Semin Thromb Hemost. 1996;22(2):203-8. doi: 10.1055/s-2007-999009. Semin Thromb Hemost. 1996. PMID: 8807718 Review.
-
Isolation and characterization of hirudin from Hirudo medicinalis.Semin Thromb Hemost. 1991 Apr;17(2):83-7. doi: 10.1055/s-2007-1002593. Semin Thromb Hemost. 1991. PMID: 1771416 Review. No abstract available.
Cited by
-
Identification and functional characterization of multiple haemadins and an oligomeric decorsin in the Asian land leech Haemadipsa interrupta.Parasitol Res. 2024 Nov 21;123(11):390. doi: 10.1007/s00436-024-08404-w. Parasitol Res. 2024. PMID: 39570434 Free PMC article.
-
Identification and Characterization of RK22, a Novel Antimicrobial Peptide from Hirudinaria manillensis against Methicillin Resistant Staphylococcus aureus.Int J Mol Sci. 2023 Aug 30;24(17):13453. doi: 10.3390/ijms241713453. Int J Mol Sci. 2023. PMID: 37686259 Free PMC article.
-
Chromosome-level genome assembly and anticoagulant protein annotation of the buffalo leech Hirudinaria bpling (Hirudinea: Hirudinidae).BMC Genomics. 2025 Jun 3;26(1):558. doi: 10.1186/s12864-025-11690-y. BMC Genomics. 2025. PMID: 40461966 Free PMC article.
References
-
- Babenko VV, Podgorny OV, Manuvera VA, Kasianov AS, Manolov AI, Grafskaia EN, Shirokov DA, Kurdyumov AS, Vinogradov DV, Nikitina AS, Kovalchuk SI, Anikanov NA, Butenko IO, Pobeguts OV, Matyushkina DS, Rakitina DV, Kostryukova ES, Zgoda VH, Baskova IP, Trukhan VM, Gelfand MS, Govorun VM, Schiöth HB, Lazarev VN. Draft genome sequences of Hirudo medicinalis and salivary transcriptome of three closely related medicinal leeches. BMC Genomics. 2020;21(1):331. doi: 10.1186/s12864-020-6748-0. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

