Non-covalent interactions from a Quantum Chemical Topology perspective
- PMID: 36006513
- PMCID: PMC9411098
- DOI: 10.1007/s00894-022-05188-7
Non-covalent interactions from a Quantum Chemical Topology perspective
Abstract
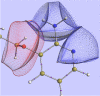
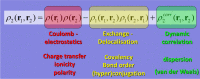
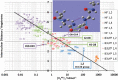
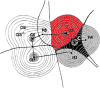
About half a century after its little-known beginnings, the quantum topological approach called QTAIM has grown into a widespread, but still not mainstream, methodology of interpretational quantum chemistry. Although often confused in textbooks with yet another population analysis, be it perhaps an elegant but somewhat esoteric one, QTAIM has been enriched with about a dozen other research areas sharing its main mathematical language, such as Interacting Quantum Atoms (IQA) or Electron Localisation Function (ELF), to form an overarching approach called Quantum Chemical Topology (QCT). Instead of reviewing the latter's role in understanding non-covalent interactions, we propose a number of ideas emerging from the full consequences of the space-filling nature of topological atoms, and discuss how they (will) impact on interatomic interactions, including non-covalent ones. The architecture of a force field called FFLUX, which is based on these ideas, is outlined. A new method called Relative Energy Gradient (REG) is put forward, which is able, by computation, to detect which fragments of a given molecular assembly govern the energetic behaviour of this whole assembly. This method can offer insight into the typical balance of competing atomic energies both in covalent and non-covalent case studies. A brief discussion on so-called bond critical points is given, highlighting concerns about their meaning, mainly in the arena of non-covalent interactions.
Keywords: Atomic electron correlation; Covalency; Exchange energy; FFLUX; Gaussian process regression; Interacting Quantum Atoms (IQA); Machine learning; Multipole moments; Polarisation; QTAIM; Quantum Chemical Topology (QCT); Relative Energy Gradient (REG).
© 2022. The Author(s).
Conflict of interest statement
The author declares no competing interests.
Figures




Similar articles
-
Probing Non-Covalent Interactions through Molecular Balances: A REG-IQA Study.Molecules. 2024 Feb 28;29(5):1043. doi: 10.3390/molecules29051043. Molecules. 2024. PMID: 38474554 Free PMC article.
-
An Interacting Quantum Atoms (IQA) and Relative Energy Gradient (REG) Study of the Halogen Bond with Explicit Analysis of Electron Correlation.Molecules. 2020 Jun 9;25(11):2674. doi: 10.3390/molecules25112674. Molecules. 2020. PMID: 32526931 Free PMC article.
-
Atomic Partitioning of the MPn (n = 2, 3, 4) Dynamic Electron Correlation Energy by the Interacting Quantum Atoms Method: A Fast and Accurate Electrostatic Potential Integral Approach.J Comput Chem. 2019 Dec 15;40(32):2793-2800. doi: 10.1002/jcc.26037. Epub 2019 Aug 2. J Comput Chem. 2019. PMID: 31373709 Free PMC article.
-
Atomic charges in molecules defined by molecular real space partition into atomic subspaces.Phys Chem Chem Phys. 2023 Mar 29;25(13):9020-9030. doi: 10.1039/d2cp05428k. Phys Chem Chem Phys. 2023. PMID: 36928882 Review.
-
A 30-Year Journey Towards an Accelerated Scheme for Visualizing ELF Basins in Molecules.J Comput Chem. 2025 Jun 15;46(16):e70146. doi: 10.1002/jcc.70146. J Comput Chem. 2025. PMID: 40485129 Review.
Cited by
-
Probing Non-Covalent Interactions through Molecular Balances: A REG-IQA Study.Molecules. 2024 Feb 28;29(5):1043. doi: 10.3390/molecules29051043. Molecules. 2024. PMID: 38474554 Free PMC article.
-
How deeply should we analyze non-covalent interactions?J Mol Model. 2023 Feb 9;29(3):66. doi: 10.1007/s00894-023-05460-4. J Mol Model. 2023. PMID: 36757533 Free PMC article.
-
3-Thiophenemalonic Acid Additive Enhanced Performance in Perovskite Solar Cells.ACS Omega. 2024 Jan 4;9(2):2674-2686. doi: 10.1021/acsomega.3c07592. eCollection 2024 Jan 16. ACS Omega. 2024. PMID: 38250358 Free PMC article.
-
Adsorption and sensor performance of transition metal-decorated zirconium-doped silicon carbide nanotubes for NO2 gas application: a computational insight.RSC Adv. 2024 Feb 12;14(8):5351-5369. doi: 10.1039/d3ra08796d. eCollection 2024 Feb 7. RSC Adv. 2024. Retraction in: RSC Adv. 2025 Apr 7;15(14):10557. doi: 10.1039/d5ra90036k. PMID: 38348297 Free PMC article. Retracted.
-
The IQA Energy Partition in a Drug Design Setting: A Hepatitis C Virus RNA-Dependent RNA Polymerase (NS5B) Case Study.Pharmaceuticals (Basel). 2022 Oct 8;15(10):1237. doi: 10.3390/ph15101237. Pharmaceuticals (Basel). 2022. PMID: 36297349 Free PMC article.
References
-
- Popelier PLA (2016) in The chemical bond - 100 years old and getting stronger, edited by M. Mingos (Springer, Switzerland), pp. 71–117
-
- Popelier PLA. in Intermolecular interactions. In: Novoa J, editor. molecular crystals. Great Britain: RSC Cambridge; 2018. pp. 147–177.
-
- Popelier PLA (2014) in The nature of the chemical bond revisited, edited by G. Frenking and S. Shaik (Wiley-VCH, Chapter 8), pp. 271–308
LinkOut - more resources
Full Text Sources

