Silica-Capped and Gold-Decorated Silica Nanoparticles for Enhancing Effect of Gold Nanoparticle-Based Photothermal Therapy
- PMID: 36006602
- PMCID: PMC9679086
- DOI: 10.1007/s13770-022-00468-y
Silica-Capped and Gold-Decorated Silica Nanoparticles for Enhancing Effect of Gold Nanoparticle-Based Photothermal Therapy
Abstract
Background: Various methods based on gold nanoparticles (AuNPs) have been applied to enhance the photothermal effect. Among these methods, combining gold nanoparticles and stem cells has been suggested as a new technique for elevating the efficiency of photothermal therapy (PT) in terms of enhancing tumor targeting effect. However, to elicit the efficiency of PT using gold nanoparticles and stem cells, delivering large amounts of AuNPs into stem cells without loss should be considered.
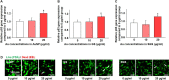
Methods: AuNPs, AuNPs-decorated silica nanoparticles, and silica-capped and AuNPs-decorated silica nanoparticles (SGSs) were synthesized and used to treat human mesenchymal stem cells (hMSCs). After evaluating physical properties of each nanoparticle, the concentration of each nanoparticle was estimated based on its cytotoxicity to hMSCs. The amount of AuNPs loss from each nanoparticle by exogenous physical stress was evaluated after exposing particles to a gentle shaking. After these experiments, in vitro and in vivo photothermal effects were then evaluated.
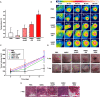
Results: SGS showed no cytotoxicity when it was used to treat hMSCs at concentration up to 20 μg/mL. After intravenous injection to tumor-bearing mice, SGS-laden hMSCs group showed significantly higher heat generation than other groups following laser irradiation. Furthermore, in vivo photothermal effect in the hMSC-SGS group was significantly enhanced than those in other groups in terms of tumor volume decrement and histological outcome.
Conclusion: Our results suggest that additional silica layer in SGSs could protect AuNPs from physical stress induced AuNPs loss. The strategy applied in SGS may offer a prospective method to improve PT.
Keywords: Gold nanoparticles; Photothermal therapy; Silica capping.
© 2022. Korean Tissue Engineering and Regenerative Medicine Society.
Conflict of interest statement
The authors declare no conflicts of interest relevant to this article.
Figures


Similar articles
-
Metal Ion Releasing Gold Nanoparticles for Improving Therapeutic Efficiency of Tumor Targeted Photothermal Therapy.Tissue Eng Regen Med. 2022 Apr;19(2):289-299. doi: 10.1007/s13770-021-00385-6. Epub 2021 Sep 24. Tissue Eng Regen Med. 2022. PMID: 34561850 Free PMC article.
-
Gold Nanoparticle-Decorated Drug Nanocrystals for Enhancing Anticancer Efficacy and Reversing Drug Resistance Through Chemo-/Photothermal Therapy.Mol Pharm. 2022 Jul 4;19(7):2518-2534. doi: 10.1021/acs.molpharmaceut.2c00150. Epub 2022 May 13. Mol Pharm. 2022. PMID: 35549267
-
Nanoscaled PAMAM Dendrimer Spacer Improved the Photothermal-Photodynamic Treatment Efficiency of Photosensitizer-Decorated Confeito-Like Gold Nanoparticles for Cancer Therapy.Macromol Biosci. 2022 Aug;22(8):e2200130. doi: 10.1002/mabi.202200130. Epub 2022 May 24. Macromol Biosci. 2022. PMID: 35579182
-
Polymer decorated gold nanoparticles in nanomedicine conjugates.Adv Colloid Interface Sci. 2017 Nov;249:386-399. doi: 10.1016/j.cis.2017.01.007. Epub 2017 Feb 15. Adv Colloid Interface Sci. 2017. PMID: 28259207 Review.
-
Towards Effective Photothermal/Photodynamic Treatment Using Plasmonic Gold Nanoparticles.Int J Mol Sci. 2016 Aug 9;17(8):1295. doi: 10.3390/ijms17081295. Int J Mol Sci. 2016. PMID: 27517913 Free PMC article. Review.
Cited by
-
Progress in Nano-Biosensors for Non-Invasive Monitoring of Stem Cell Differentiation.Biosensors (Basel). 2023 Apr 26;13(5):501. doi: 10.3390/bios13050501. Biosensors (Basel). 2023. PMID: 37232862 Free PMC article. Review.
-
Recent Advances in Aptamer-Based Sensors for Sensitive Detection of Neurotransmitters.Biosensors (Basel). 2023 Mar 23;13(4):413. doi: 10.3390/bios13040413. Biosensors (Basel). 2023. PMID: 37185488 Free PMC article. Review.
-
Review of Advances in Coating and Functionalization of Gold Nanoparticles: From Theory to Biomedical Application.Pharmaceutics. 2024 Feb 9;16(2):255. doi: 10.3390/pharmaceutics16020255. Pharmaceutics. 2024. PMID: 38399309 Free PMC article. Review.
-
Nanomaterial-based detection of circulating tumor cells and circulating cancer stem cells for cancer immunotherapy.Nano Converg. 2024 Dec 13;11(1):56. doi: 10.1186/s40580-024-00466-x. Nano Converg. 2024. PMID: 39671082 Free PMC article. Review.
-
Recent Studies on Metal-Embedded Silica Nanoparticles for Biological Applications.Nanomaterials (Basel). 2024 Jan 26;14(3):268. doi: 10.3390/nano14030268. Nanomaterials (Basel). 2024. PMID: 38334538 Free PMC article. Review.
References
-
- Roh YH, Eom JY, Choi DG, Moon JY, Shim MS, Bong KW. Gold nanorods-encapsulated thermosensitive drug carriers for NIR light-responsive anticancer therapy. J Ind Eng Chem. 2021;98:211–216. doi: 10.1016/j.jiec.2021.03.052. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
