Therapeutic Management of Idiosyncratic Drug-Induced Liver Injury and Acetaminophen Hepatotoxicity in the Paediatric Population: A Systematic Review
- PMID: 36006605
- PMCID: PMC9560995
- DOI: 10.1007/s40264-022-01224-w
Therapeutic Management of Idiosyncratic Drug-Induced Liver Injury and Acetaminophen Hepatotoxicity in the Paediatric Population: A Systematic Review
Abstract
Introduction: Drug-induced liver injury (DILI) is a rare but serious adverse event that can progress to acute liver failure (ALF). The evidence for treatment of DILI in children is scarce.
Objective: We aimed to comprehensively review the available literature on the therapies for both acetaminophen overdose (APAP) and idiosyncratic DILI in the paediatric population.
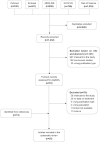
Methods: We included original articles conducted in a paediatric population (< 18 years) in which a therapeutic intervention was described to manage APAP or idiosyncratic DILI. Findings were summarized based on age groups (preterm newborn neonates, term and post-term neonates, infants, children and adolescents).
Results: Overall, 25 publications (fifteen case reports, six case series and four retrospective cohort studies) were included, including a total of 140 paediatric DILI cases, from preterm newborn neonates to adolescents. N-acetylcysteine was used to treat 19 APAP cases. N-acetylcysteine (n = 14), ursodeoxycholic acid (n = 3), corticosteroids (n = 31), carnitine (n = 16) and the combination of glycyrrhizin, reduced glutathione, polyene phosphatidylcholine and S-adenosylmethionine (n = 31) were the therapeutic options for treating idiosyncratic DILI. The molecular adsorbent recirculating system was used in the management of either APAP (n = 4) or idiosyncratic DILI (n = 2), while 20 paediatric ALF cases received continuous renal replacement therapy.
Conclusions: This systematic review identified DILI in the paediatric population who have received specific treatment. These interventions appear to be mainly extrapolated from low-quality evidence from the adult population. Thus, there is a need for high-quality studies to test the efficacy of known and novel therapies to treat DILI specifically addressed to the paediatric population. PROSPERO registration number CRD42021214702.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Acetaminophen: Dose-Dependent Drug Hepatotoxicity and Acute Liver Failure in Patients.Dig Dis. 2015;33(4):464-71. doi: 10.1159/000374090. Epub 2015 Jul 6. Dig Dis. 2015. PMID: 26159260 Free PMC article. Review.
-
Acetaminophen-related hepatotoxicity.Clin Liver Dis. 2013 Nov;17(4):587-607, viii. doi: 10.1016/j.cld.2013.07.005. Epub 2013 Sep 4. Clin Liver Dis. 2013. PMID: 24099020 Review.
-
The diagnosis and management of idiosyncratic drug-induced liver injury.Liver Int. 2019 Jan;39(1):31-41. doi: 10.1111/liv.13931. Epub 2018 Aug 19. Liver Int. 2019. PMID: 30003672 Review.
-
Prevention and management of idiosyncratic drug-induced liver injury: Systematic review and meta-analysis of randomised clinical trials.Pharmacol Res. 2021 Feb;164:105404. doi: 10.1016/j.phrs.2020.105404. Epub 2020 Dec 24. Pharmacol Res. 2021. PMID: 33359912
-
Evaluation of drug-induced liver injury as etiology for acute liver failure in Brazil.Ann Hepatol. 2021 Jul-Aug;23:100310. doi: 10.1016/j.aohep.2021.100310. Epub 2021 Jan 27. Ann Hepatol. 2021. PMID: 33508520
Cited by
-
Toxicity of targeted anticancer treatments on the liver in myeloproliferative neoplasms.World J Hepatol. 2023 Sep 27;15(9):1021-1032. doi: 10.4254/wjh.v15.i9.1021. World J Hepatol. 2023. PMID: 37900211 Free PMC article. Review.
-
Repurposing N-acetylcysteine for management of non-acetaminophen induced acute liver failure: an evidence scan from a global health perspective.Transl Gastroenterol Hepatol. 2024 Jan 18;9:2. doi: 10.21037/tgh-23-40. eCollection 2024. Transl Gastroenterol Hepatol. 2024. PMID: 38317753 Free PMC article.
-
Drug-induced liver injury in children: A nationwide cohort study from China.JHEP Rep. 2024 Apr 25;6(8):101102. doi: 10.1016/j.jhepr.2024.101102. eCollection 2024 Aug. JHEP Rep. 2024. PMID: 39105181 Free PMC article.
-
Molecular Adsorbent Recirculating System for Acute Liver Failure in a New Pediatric-Based Extracorporeal Liver Support Program.Crit Care Explor. 2023 Nov 8;5(11):e1002. doi: 10.1097/CCE.0000000000001002. eCollection 2023 Nov. Crit Care Explor. 2023. PMID: 37954902 Free PMC article.
-
Roadmap to DILI research in Europe. A proposal from COST action ProEuroDILINet.Pharmacol Res. 2024 Feb;200:107046. doi: 10.1016/j.phrs.2023.107046. Epub 2023 Dec 28. Pharmacol Res. 2024. PMID: 38159783 Free PMC article.

