Comparing Kidney Health Outcomes in Children, Adolescents, and Adults With Focal Segmental Glomerulosclerosis
- PMID: 36006643
- PMCID: PMC9412226
- DOI: 10.1001/jamanetworkopen.2022.28701
Comparing Kidney Health Outcomes in Children, Adolescents, and Adults With Focal Segmental Glomerulosclerosis
Abstract
Importance: Focal segmental glomerulosclerosis (FSGS) is a common cause of end-stage kidney disease (ESKD) across the lifespan. While 10% to 15% of children and 3% of adults who develop ESKD have FSGS, it remains uncertain whether the natural history differs in pediatric vs adult patients, and this uncertainty contributes to the exclusion of children and adolescents in clinical trials.
Objective: To examine whether there are differences in the kidney health outcomes among children, adolescents, and adults with FSGS.
Design, setting, and participants: This cohort study used pooled and parallel analyses, completed July 5, 2022, from 3 complimentary data sources: (1) Nephrotic Syndrome Rare Disease Clinical Research Network (NEPTUNE); (2) FSGS clinical trial (FSGS-CT); and (3) Kidney Research Network (KRN). NEPTUNE is a multicenter US/Canada cohort study; FSGS-CT is a multicenter US/Canada clinical trial; and KRN is a multicenter US electronic health record-based registry from academic and community nephrology practices. NEPTUNE included 166 patients with incident FSGS enrolled at first kidney biopsy; FSGS-CT included 132 patients with steroid-resistant FSGS randomized to cyclosporine vs dexamethasone with mycophenolate; and KRN included 184 patients with prevalent FSGS. Data were collected from November 2004 to October 2019 and analyzed from October 2020 to July 2022.
Exposures: Age: children (age <13 years) vs adolescents (13-17 years) vs adults (≥18 years). Covariates of interest included sex, disease duration, APOL1 genotype, urine protein-to-creatinine ratio, estimated glomerular filtration rate (eGFR), edema, serum albumin, and immunosuppressive therapy.
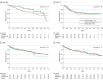
Main outcomes and measures: ESKD, composite outcome of ESKD or 40% decline in eGFR, and complete and/or partial remission of proteinuria.
Results: The study included 127 (26%) children, 102 (21%) adolescents, and 253 (52%) adults, including 215 (45%) female participants and 138 (29%) who identified as Black, 98 (20%) who identified as Hispanic, and 275 (57%) who identified as White. Overall, the median time to ESKD was 11.9 years (IQR, 5.2-19.1 years). There was no difference in ESKD risk among children vs adults (hazard ratio [HR], 0.67; 95% CI, 0.43-1.03) or adolescents vs adults (HR, 0.85; 95% CI, 0.52-1.36). The median time to the composite end point was 5.7 years (IQR 1.6-15.2 years), with hazard ratio estimates for children vs adults of 1.12 (95% CI, 0.83-1.52) and adolescents vs adults of 1.06 (95% CI, 0.75-1.50).
Conclusions and relevance: In this study, the association of FSGS with kidney survival and functional outcomes was comparable at all ages.
Conflict of interest statement
Figures



References
-
- Baigent C, Herrington WG, Coresh J, et al. ; KDIGO Controversies Conference on Challenges in the Conduct of Clinical Trials in Nephrology Conference Participants . Challenges in conducting clinical trials in nephrology: conclusions from a Kidney Disease-Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2017;92(2):297-305. doi: 10.1016/j.kint.2017.04.019 - DOI - PMC - PubMed
-
- Gaspar N, Marshall LV, Binner D, et al. ; Members of Working Group 1 of the Paediatric Platform of ACCELERATE . Joint adolescent-adult early phase clinical trials to improve access to new drugs for adolescents with cancer: proposals from the multi-stakeholder platform-ACCELERATE. Ann Oncol. 2018;29(3):766-771. doi: 10.1093/annonc/mdy002 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

