Guidelines for Artificial Intelligence in Medicine: Literature Review and Content Analysis of Frameworks
- PMID: 36006692
- PMCID: PMC9459836
- DOI: 10.2196/36823
Guidelines for Artificial Intelligence in Medicine: Literature Review and Content Analysis of Frameworks
Abstract
Background: Artificial intelligence (AI) is rapidly expanding in medicine despite a lack of consensus on its application and evaluation.
Objective: We sought to identify current frameworks guiding the application and evaluation of AI for predictive analytics in medicine and to describe the content of these frameworks. We also assessed what stages along the AI translational spectrum (ie, AI development, reporting, evaluation, implementation, and surveillance) the content of each framework has been discussed.
Methods: We performed a literature review of frameworks regarding the oversight of AI in medicine. The search included key topics such as "artificial intelligence," "machine learning," "guidance as topic," and "translational science," and spanned the time period 2014-2022. Documents were included if they provided generalizable guidance regarding the use or evaluation of AI in medicine. Included frameworks are summarized descriptively and were subjected to content analysis. A novel evaluation matrix was developed and applied to appraise the frameworks' coverage of content areas across translational stages.
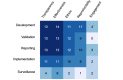
Results: Fourteen frameworks are featured in the review, including six frameworks that provide descriptive guidance and eight that provide reporting checklists for medical applications of AI. Content analysis revealed five considerations related to the oversight of AI in medicine across frameworks: transparency, reproducibility, ethics, effectiveness, and engagement. All frameworks include discussions regarding transparency, reproducibility, ethics, and effectiveness, while only half of the frameworks discuss engagement. The evaluation matrix revealed that frameworks were most likely to report AI considerations for the translational stage of development and were least likely to report considerations for the translational stage of surveillance.
Conclusions: Existing frameworks for the application and evaluation of AI in medicine notably offer less input on the role of engagement in oversight and regarding the translational stage of surveillance. Identifying and optimizing strategies for engagement are essential to ensure that AI can meaningfully benefit patients and other end users.
Keywords: AI; artificial intelligence; effectiveness; engagement; ethics; health care; medicine; reproducibility; translational research; translational science; transparency.
©Norah L Crossnohere, Mohamed Elsaid, Jonathan Paskett, Seuli Bose-Brill, John F P Bridges. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 25.08.2022.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Rivera SC, Liu X, Chan A, Denniston AK, Calvert MJ, SPIRIT-AICONSORT-AI Working Group Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI Extension. BMJ. 2020 Sep 09;370:m3210. doi: 10.1136/bmj.m3210. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=32907797 - DOI - PMC - PubMed
-
- Artificial Intelligence in Healthcare Market by Offering, Technology, Application, End User and Geography - Global Forecast to 2027. ReportLinker. 2021. Oct, [2021-07-14]. https://tinyurl.com/4dh7bdn7 .
-
- Amisha PM, Malik P, Pathania M, Rathaur V. Overview of artificial intelligence in medicine. J Family Med Prim Care. 2019 Jul;8(7):2328–2331. doi: 10.4103/jfmpc.jfmpc_440_19. http://www.jfmpc.com/article.asp?issn=2249-4863;year=2019;volume=8;issue... JFMPC-8-2328 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

