Memantine for autism spectrum disorder
- PMID: 36006807
- PMCID: PMC9409629
- DOI: 10.1002/14651858.CD013845.pub2
Memantine for autism spectrum disorder
Abstract
Background: Autism spectrum disorder (ASD; also known as autism) is a developmental disability that begins in childhood and is typically seen in around 1% to 2% of children. It is characterised by social communication difficulties and repetitive and restricted behaviours and routines that can have a negative impact on a child's quality of life, achievement at school, and social interactions with others. It has been hypothesised that memantine, which is traditionally used to treat dementia, may be effective in reducing the core symptoms of autism as well as some co-occurring symptoms such as hyperactivity and language difficulties. If memantine is being used to treat the core symptoms of autism, it is important to review the evidence of its effectiveness.
Objectives: To assess the effects of memantine on the core symptoms of autism, including, but not limited to, social communication and stereotypical behaviours.
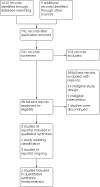
Search methods: We searched CENTRAL, MEDLINE, Embase, nine other databases and three trials registers up to February 2022. We also checked reference lists of key studies and checked with experts in the field for any additional papers. We searched for retractions of the included studies in MEDLINE, Embase, and the Retraction Watch Database. No retractions or corrections were found.
Selection criteria: We included randomised controlled trials (RCTs) of any dose of memantine compared with placebo in autistic people. We also included RCTs in which only one group received memantine, but both groups received the same additional therapy (e.g. a behaviour intervention).
Data collection and analysis: We used standard Cochrane methods. Our primary outcomes were core autism symptoms and adverse effects. Secondary outcomes were language, intelligence, memory, adaptive behaviour, hyperactivity, and irritability. We used GRADE to assess certainty of evidence.
Main results: We included three RCTs (two double-blind and one single-blind) with 204 participants that examined the short-term effect (immediately postintervention) of memantine in autistic people. Two studies took place in the USA and the other in Iran. All three studies focused on children and adolescents, with a mean age of 9.40 (standard deviation (SD) 2.26) years. Most participants were male (range across studies 73% to 87%). The diagnosis of ASD was based on the Diagnostic and Statistical Manual of Mental Disorders (4th edition; 4th edition, text revision; or 5th edition). To confirm the diagnosis, one study used the Autism Diagnostic Observation Schedule (ADOS) and the Autism Diagnostic Interview-Revised (ADI-R); one used ADOS, ADI-R or the Autism Diagnostic Interview Screener; and one used the Gilliam Autism Rating Scale. Dosage of memantine was based on the child's weight and ranged from 3 mg to 15 mg per day. Comparisons Two studies examined memantine compared with placebo; in the other study, both groups had a behavioural intervention while only one group was given memantine. Risk of bias All studies were rated at high risk of bias overall, as they were at high or unclear risk of bias across all but four domains in one study, and all but two domains in the other two studies. One study was funded by Forest Laboratories, LLC, (Jersey City, New Jersey), Allergan. The study sponsor was involved in the study design, data collection (via contracted clinical investigator sites), analysis and interpretation of data, and the decision to present these results. The other two studies reported no financial support or sponsorship; though in one of the two, the study medication was an in-kind contribution from Forest Pharmaceuticals. Primary outcomes There was no clear evidence of a difference between memantine and placebo with respect to severity of core symptoms of autism, although we are very uncertain about the evidence. The standardised mean difference in autism symptoms score in the intervention group versus the control group was -0.74 standard deviations (95% confidence interval (CI) -2.07 to 0.58; 2 studies, 181 participants; very low-certainty evidence; medium effect size); lower scores indicate less severe autistic symptoms. Two studies (144 participants) recorded adverse effects that the authors deemed related to the study and found there may be no difference between memantine and placebo (odds ratio (OR) 0.64, 95% CI 0.17 to 2.39; low-certainty evidence). Secondary outcomes There may be no difference between memantine and placebo on language (2 studies, 144 participants; low-certainty evidence); memory or adaptive behaviour (1 study, 23 participants; both low-certainty evidence); or hyperactivity or irritability (1 study, 121 participants; both low-certainty evidence).
Authors' conclusions: It is unclear whether memantine is an effective treatment for autistic children. None of the three included trials reported on the effectiveness of memantine in adults. Further studies using rigorous designs, larger samples, longer follow-up and clinically meaningful outcome measures that are important to autistic people and their families will strengthen our knowledge of the effects of memantine in autism.
Trial registration: ClinicalTrials.gov NCT00872898 NCT01372449 NCT01592747 NCT01592773 NCT01078844 NCT02353130 NCT01972074 NCT03553875.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
AB is a Speech Pathologist in developmental paediatrics at Monash Children's Hospital; her role is assessment and not ongoing management. AB is also an Associate Editor with Cochrane Developmental, Psychosocial and Learning Problems (DPLP).
CM is a Consultant Paediatrician at the Royal Children's Hospital Melbourne, where she is involved in both clinical care and research with pharmacological and non‐pharmacological interventions for individuals with ASD. CM has recently received commercial funding paid to her research institute (MCRI) to establish safety and efficacy for a drug called AB‐2004 for children with ASD (Axial Therapeutics). She has received no commercial funding for clinical care.
KW is a Paediatrician in developmental paediatrics at Monash Children's Hospital; her role is assessment and not ongoing management. KW reports a grant from the National Health And Medical Research Council (NHMRC) for a phase III trial of cannabidiol for severe behaviour disorder in children with an intellectual disability, with or without autism, on which she is Chief Investigator (1 January 2020 to 31 May 2023); this grant was paid to the institution, but KW benefited from the payment or had access to the funds. KW also reports contracts with Epsilon Healthcare (formerly THC Global Group Ltd; ongoing since 1 January 2020) to develop an interventional product and placebo for children, which was provided by the Victorian Government, for a Medical Research Future Fund funded phase III multisite trial; and with Tilray (from 28 November 2018 to 27 November 2019) for the investigation drug and placebo for a pilot trial of cannabidiol for severe behaviour problems in children with intellectual disability, with or without autism; both paid to institution. KW reports a grant from the NHMRC for an ongoing prognosis study about predictors of autism outcome that will also publish diagnostic stability outcomes and that could be included in an update of this systematic review; paid to institution. Lastly, KW reports that she is an Editor with DPLP.
TM is a Senior Research Fellow at Monash University and a Psychologist in private practice, involved in both clinical care and research with non‐pharmacological interventions for individuals with autism.
Figures
Update of
- doi: 10.1002/14651858.CD013845
References
References to studies included in this review
Aman 2017a {published data only}
-
- Aman MG, Findling RL, Hardan AY, Hendren RL, Melmed RD, Kehinde-Nelson O, et al. Safety and efficacy of memantine in children with autism: randomized, placebo-controlled study and open-label extension. Journal of Child and Adolescent Psychopharmacology 2017;27(5):403-12. [DOI: 10.1089/cap.2015.0146] [PMCID: PMC5510039] [PMID: ] - DOI - PMC - PubMed
-
- NCT00872898. Study of pharmacokinetics, safety, efficacy, and tolerability of memantine in children with autism [An open-label (part one) and a randomized, double-blind, placebo-controlled (part two) study of the pharmacokinetics, safety, efficacy, and tolerability of memantine in pediatric patients with autism]. clinicaltrials.gov/ct2/show/NCT00872898 (first received 31 March 2009).
Karahmadi 2018 {published data only}
Soorya 2021 {published data only}
-
- Bader YK. A double-blind placebo-controlled trial of memantine vs placebo in children with autism spectrum disorder (ASD) targeting memory and motor planning [MS thesis]. Ann Arbor (MI): Rush University, 2016.
-
- NCT01372449. A multi-site double-blind placebo-controlled trial of memantine versus placebo in children with autism (MEM) [A multi-site double-blind placebo-controlled trial of memantine versus placebo in children with autism targeting memory and motor planning]. clinicaltrials.gov/ct2/show/NCT01372449 (first received 14 June 2011).
-
- Soorya LV, Fogg L, Ocampo E, Printen M, Youngkin S, Halpern D, et al. Neurocognitive outcomes from memantine: a pilot, double-blind, placebo-controlled trial in children with autism spectrum disorder. Journal of Child and Adolescent Psychopharmacology 2021;31(7):475-84. [DOI: 10.1089/cap.2021.0010] [PMID: ] - DOI - PubMed
References to studies excluded from this review
Ghaleiha 2013 {published data only}IRCT1138901151556N10
-
- Ghaleiha A, Asadabadi M, Mohammadi M-R, Shahei M, Tabrizi M, Hajiaghaee R, et al. Memantine as adjunctive treatment to risperidone in children with autistic disorder: a randomized, double-blind, placebo-controlled trial. International Journal of Neuropsychopharmacology 2013;16(4):783-9. [DOI: 10.1017/S1461145712000880] - DOI - PubMed
-
- IRCT1138901151556N10. Memantine in the treatment of autism [A double- blind placebo controlled trial of memantine added to risperidone in patients with autistic disorder]. www.irct.ir/trial/860 (first received 1 May 2010).
Hardan 2019 {published data only}
-
- EUCTR 2012-001568-31-BE. A double-blind, placebo-controlled, randomized withdrawal study of the safety and efficacy of memantine in pediatric patients with autism, Asperger's disorder, or pervasive developmental disorder not otherwise specified (PDD-NOS) previously treated with memantine. www.clinicaltrialsregister.eu/ctr-search/trial/2012-001568-31/BE (first received 19 July 2012).
-
- Hardan AY, Hendren RL, Aman MG, Robb A, Melmed RD, Andersen KA, et al. Efficacy and safety of memantine in children with autism spectrum disorder: results from three phase 2 multicenter studies. Autism 2019;23(8):2096-111. [DOI: 10.1177/1362361318824103] [PMCID: PMC6779018] [PMID: ] - DOI - PMC - PubMed
-
- NCT01592747. Withdrawal study of memantine in pediatric patients with autism, Asperger's disorder, or pervasive developmental disorder not otherwise specified previously treated with memantine [A double-blind, placebo-controlled, randomised withdrawal study of the safety and efficacy of memantine in pediatric patients with autism, Asperger's disorder, or pervasive developmental disorder not otherwise specified previously treated with memantine]. clinicaltrials.gov/ct2/show/NCT01592747 (first received 7 May 2012).
-
- NCT01592773. Safety study of memantine in pediatric patients with autism, Asperger's disorder or pervasive developmental disorder not otherwise specified (PDD-NOS) [An open-label extension study of the safety and tolerability of memantine in pediatric patients with autism, Asperger's disorder, or pervasive developmental disorder not otherwise specified (PDD-NOS)]. clinicaltrials.gov/ct2/show/NCT01592773 (first received 7 May 2012).
NCT01078844 {published data only}
-
- NCT01078844. Memantine in adult autism spectrum disorder. clinicaltrials.gov/ct2/show/NCT01078844 (first received 2 March 2010).
NCT02353130 {published data only}
-
- NCT02353130. Cortical metrics assessment outcome measure development in autism with memantine treatment [Development of cortical metrics assessment outcome measures in response to memantine treatment in autism spectrum disorders]. clinicaltrials.gov/ct2/show/NCT02353130 (first received 2 February 2015).
References to studies awaiting assessment
Martsenkovsky 2016 {published data only}
-
- Martsenkovsky I, Martsenkovska I. The safety and efficacy of memantine hydrochloride versus placebo for children under 3 years old with autism spectrum disorders. European Neuropsychopharmacology 2016;26(Suppl 2):S729. [DOI: 10.1016/S0924-977X(16)31879-X] - DOI
References to ongoing studies
EUCTR 2014‐003080‐38‐DE {published data only}
-
- EUCTR 2014-003080-38-DE. Glutamatergic medication in the treatment of obsessive compulsive disorder (OCD) and autism spectrum disorder (ASD). www.clinicaltrialsregister.eu/ctr-search/trial/2014-003080-38/DE (first received 22 December 2014).
-
- Häge A, Banaschewski T, Buitelaar JK, Dijkhuizen RM, Franke B, Lythgoe DJ, et al. Glutamatergic medication in the treatment of obsessive compulsive disorder (OCD) and autism spectrum disorder (ASD) – study protocol for a randomised controlled trial. Trials 2016;17(1 ):141. [DOI: 10.1186/s13063-016-1266-8] [PMCID: PMC4794817] [PMID: ] - DOI - PMC - PubMed
NCT01972074 {published data only}
-
- NCT01972074. Behavioral and neural response to memantine in adolescents with autism spectrum disorder. clinicaltrials.gov/ct2/show/NCT01972074 (first received 30 October 2013).
NCT03553875 {published data only}
-
- NCT03553875. Memantine for the treatment of social deficits in youth with disorders of impaired social interactions [Memantine for the treatment of social deficits in youth with disorders of impaired social interactions: a randomized-controlled trial]. clinicaltrials.gov/ct2/show/NCT03553875 (first received 12 June 2018).
Additional references
Aman 2017b
-
- Aman MG, Singh NN. Aberrant Behavior Checklist Manual. 2nd edition. East Aurora, NY: Slosson Educational Publications, 2017.
Anagnostou 2015
APA 1980
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III). 3rd edition. Washington (DC): American Psychiatric Association, 1980.
APA 1987
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R). 3rd edition, revised. American Psychiatric Association, 1987.
APA 1994
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th edition. Washington (DC): American Psychiatric Association, 1994.
APA 2000
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). 4th edition, text revision. Washington (DC): American Psychiatric Association, 2000.
APA 2013
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th edition. Arlington (VA): American Psychiatric Association, 2013.
Aye 2021
-
- Aye SZ, Ni H, Sein HH, Mon ST, Zheng Q, Wong YK. The effectiveness and adverse effects of D‐cycloserine compared with placebo on social and communication skills in individuals with autism spectrum disorder. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD013457. [DOI: 10.1002/14651858.CD013457.pub2] [PMCID: PMC8094878 (available on 14 February 2022)] [PMID: ] - DOI - PMC - PubMed
Bayou 2008
-
- Bayou N, M'Rad R, Ahlem B, Béchir Helayem M, Chaabouni H. Autism: an overview of genetic aetiology. La Tunisie Médicale 2008;86(6):573-8. [PMID: ] - PubMed
Beach Center on Disabilities 2006
-
- Beach Center on Disabilities. Family Quality of Life Scale. Lawrence (KS): Beach Center on Disabilities, 2006.
Becker 2013
Begg 1994
Biederman 2017
-
- Biederman J, Fried R, Tarko L, Surman C, Spencer T, Pope A, et al. Memantine in the treatment of executive function deficits in adults with ADHD: a pilot-randomized double-blind controlled clinical trial. Journal of Attention Disorders 2017;21(4):343-52. [DOI: 10.1177/1087054714538656] [PMID: ] - DOI - PubMed
Bourke 2016
-
- Bourke J, De Klerk N, Smith T, Leonard H. Population-based prevalence of intellectual disability and autism spectrum disorders in western Australia: a comparison with previous estimates. Medicine 2016;95(21):e3737. [DOI: 10.1097/MD.0000000000003737] [PMCID: PMC4902360 ] [PMID: ] - DOI - PMC - PubMed
Caddy 2015
Castelbaum 2020
Cheuk 2011
Chez 2007
-
- Chez MG, Burton Q, Dowling T, Chang M, Khanna P, Kramer C. Memantine as adjunctive therapy in children diagnosed with autistic spectrum disorders: an observation of initial clinical response and maintenance tolerability. Journal of Child Neurology 2007;22(5):574-9. [DOI: 10.1177/0883073807302611] [PMID: ] - DOI - PubMed
Cohen 1988
-
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Erlbaum, 1988. [ISBN: 0-8058-0283-5]
Conners 1997
-
- Conners CK. Conners' Rating Scales – Revised. North Tonawanda (NY): Multi-Health Systems, Inc, 1997.
Constantino 2011
-
- Constantino J. Social Responsiveness Scale. 2nd edition. Los Angeles (CA): Western Psychological Services, 2011.
Covidence 2020 [Computer program]
-
- Covidence 2020. Version accessed 20 December 2020. Melbourne, Australia: Veritas Health Innovation, 2020. Available at covidence.org.
Deeks 2021
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
DerSimonian 1986
DerSimonian 2015
Doyle 2012
Dunn 2018
-
- Dunn D. Peabody Picture Vocabulary Test. 5th edition. Minneapolis (MN): Pearson, 2018.
Ecker 2017
Egger 1997
Elsabbagh 2012
Erickson 2007
Farlow 2008
Farmer 2013
Feusner 2009
-
- Feusner JD, Kerwin L, Saxena S, Bystritsky A. Differential efficacy of memantine for obsessive-compulsive disorder vs generalized anxiety disorder: an open-label trial. Psychopharmacology Bulletin 2009;42(1):81-93. [PMID: ] - PubMed
Findling 2007
-
- Findling RL, McNamara NK, Stansbrey RJ, Maxhimer R, Periclou A, Mann A, et al. A pilot evaluation of the safety, tolerability, pharmacokinetics, and effectiveness of memantine in pediatric patients with attention-deficit/hyperactivity disorder combined type. Journal of Child Adolescent Psychopharmacology 2007;17(1):19-33. [DOI: 10.1089/cap.2006.0044] [PMID: ] - DOI - PubMed
Fisch 2012
Fombonne 2011
-
- Fombonne E, Quirke S, Hagen A. Epidemiology of pervasive developmental disorders. In: Amaral DG, Dawson G, Geschwind DH, editors(s). Autism Spectrum Disorders. Oxford (UK): Oxford University Press, 2011:90-111.
Fombonne 2021
Gotham 2015
-
- Gotham K, Marvin AR, Taylor JL, Warren Z, Anderson CM, Law PA, et al. Characterizing the daily life, needs, and priorities of adults with autism spectrum disorder from Interactive Autism Network data. Autism 2015;19(7):794-804. [DOI: 10.1177/1362361315583818] [PMCID: PMC4581903] [PMID: ] - DOI - PMC - PubMed
GRADEpro 2020 [Computer program]
-
- GRADEpro GDT. Version accessed 20 October 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Guy 1976
-
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville (MD): US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs, 1976.
Haghighi 2013
-
- Haghighi M, Jahangard L, Mohammad-Beigi H, Bajoghli H, Hafezian H, Rahimi A, et al. In a double-blind, randomized and placebo-controlled trial, adjuvant memantine improved symptoms in inpatients suffering from refractory obsessive-compulsive disorders (OCD). Psychopharmacology 2013;228(4):633-40. [DOI: 10.1007/s00213-013-3067-z] [PMID: ] - PubMed
Hallmayer 2011
-
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry 2011;68(11):1095-102. [DOI: 10.1001/archgenpsychiatry.2011.76] [PMCID: PMC4440679] [PMID: ] - DOI - PMC - PubMed
Henneberry 2021
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1.
Higgins 2021a
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Higgins 2021b
-
- Higgins JP, Li T, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Hosenbocus 2013
James 2011
James 2015
Kavirajan 2009
Kenny 2016
Korkman 2007
-
- Korkman M, Kirk U, Kemp S. NEPSY-II. 2nd edition. San Antonio (TX): Psychological Assessment, 2007.
Kornhuber 2007
Lai 2014
Lefebvre 2021
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Li 2021
-
- Li T, Higgins JP, Deeks JJ, editor(s). Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Loomes 2017
Lord 1994
-
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview – Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders 1994;24(5):659-85. [DOI: 10.1007/BF02172145] [PMID: ] - DOI - PubMed
Lord 2012
-
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. ADOS-2: Autism Diagnostic Observation Schedule, Manual (Part 1): Modules 1–4. 2nd edition. Torrance (CA): Western Psychological Services, 2012.
Maenner 2021
-
- Maenner MJ, Shaw KA, Bakian AV, Bilder DA, Durkin MA, Esler A, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR Surveillance Summaries 2021;70(11):1–16. [DOI: 10.15585/mmwr.ss7011a1] [PMCID: PMC8639024] [PMID: ] - DOI - PMC - PubMed
Maski 2011
May 2020
McCloud 2015
-
- McCloud TL, Caddy C, Jochim J, Rendell JM, Diamond PR, Shuttleworth C, et al. Ketamine and other glutamate receptor modulators for depression in bipolar disorder in adults. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD011611. [DOI: 10.1002/14651858.CD011611.pub2] [PMID: ] - DOI - PubMed
McConachie 2015
McKay 2003
McShane 2019
Millward 2008
Mohammadi 2015
Mohammadzadeh 2019
Moher 2009
Nichols 2008
Niederhofer 2007
Nye 2005
Ott 2007
-
- Ott BR, Blake LM, Kagan E, Resnick M, Memantine MEM-MD-11AB Study Group. Open label, multicenter, 28-week extension study of the safety and tolerability of memantine in patients with mild to moderate Alzheimer's disease. Journal of Neurology 2007;254(3):351-8. [DOI: 10.1007/s00415-006-0374-x] [PMID: ] - DOI - PubMed
Owley 2006
-
- Owley T, Salt J, Guter S, Grieve A, Walton L, Ayuyao N, et al. A prospective, open-label trial of memantine in the treatment of cognitive, behavioral, and memory dysfunction in pervasive developmental disorders. Journal of Child and Adolescent Psychopharmacology 2006;16(5):517-24. [DOI: 10.1089/cap.2006.16.517] [PMID: ] - DOI - PubMed
Payakachat 2012
-
- Payakachat N, Tilford JM, Kovacs E, Kuhlthau K. Autism spectrum disorders: a review of measures for clinical, health services and cost-effectiveness applications. Expert Review of Pharmacoeconomics & Outcomes Research 2012;12(4):485-503. [DOI: 10.1586/erp.12.29] [PMCID: PMC3502071] [PMID: ] - DOI - PMC - PubMed
Rabins 2007
-
- Rabins PV, Blacker D, Rovner BW, Rummans T, et al, APA Work Group on Alzheimer's Disease and other Dementias. American Psychiatric Association practice guideline for the treatment of patients with Alzheimer's disease and other dementias. 2nd edition. American Journal of Psychiatry 2007;164(12 Suppl):5-56. [PMID: ] - PubMed
Rammes 2001
-
- Rammes G, Rupprecht R, Ferrari U, Zieglgänsberger W, Parsons CG. The N-methyl-D-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner. Neuroscience Letters 2001;306(1-2):81-4. [DOI: 10.1016/s0304-3940(01)01872-9] [PMID: ] - DOI - PubMed
Rapp 2013
Reiser 1988
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rojas 2014
Rossignol 2014
Sani 2012
-
- Sani G, Serra G, Kotzalidis GD, Romano S, Tamorri SM, Manfredi G, et al. The role of memantine in the treatment of psychiatric disorders other than the dementias: a review of current preclinical and clinical evidence. CNS Drugs 2012;26(8):663-90. [DOI: 10.2165/11634390-000000000-00000] [PMID: ] - DOI - PubMed
Scahill 2015
Schopler 1986
-
- Schopler E, Reichler RJ, Renner BR. The Childhood Autism Rating Scale (CARS): For Diagnostic Screening and Classification of Autism. New York (NY): Irvington, 1986.
Schünemann 2021
-
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Schwartz 2012
Seeman 2008
Shattuck 2007
-
- Shattuck PT, Seltzer MM, Greenberg JS, Orsmond GI, Bolt D, Kring S, et al. Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. Journal of Autism and Developmental Disorders 2007;37(9):1735-47. [DOI: 10.1007/s10803-006-0307-7] [PMCID: PMC3265360] [PMID: ] - DOI - PMC - PubMed
Siegel 2012
Sinha 2011
Smith 2013
-
- Smith EG, Deligiannidis KM, Ulbricht CM, Landolin CS, Patel JK, Rothschild AJ. Antidepressant augmentation using the N-methyl-D-aspartate antagonist memantine: a randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychiatry 2013;74(10):966-73. [DOI: 10.4088/JCP.12m08252] [PMCID: PMC4000742] [PMID: ] - DOI - PMC - PubMed
Sparrow 2016
-
- Sparrow SS, Cicchetti DV, Saulnier CA. Vineland-3: Vineland Adaptive Behavior Scales. 3rd edition. San Antonio (TX): Pearson, 2016.
Stewart 2010
Strzelecki 2013
-
- Strzelecki D, Tabaszewska A, Barszcz Z, Józefowicz O, Kropiwnicki P, Rabe-Jabłońska J. A 10-week memantine treatment in bipolar depression: a case report. Focus on depressive symptomatology, cognitive parameters and quality of life. Psychiatry Investigation 2013;10(4):421-4. [DOI: 10.4306/pi.2013.10.4.421] [PMCID: PMC3902162] [PMID: ] - DOI - PMC - PubMed
Surman 2013
Thomas 2009
Uzunova 2014
-
- Uzunova G, Hollander E, Shepherd J. The role of ionotropic glutamate receptors in childhood neurodevelopmental disorders: autism spectrum disorders and fragile x syndrome. Current Neuropharmacology 2014;12(1):71-98. [DOI: 10.2174/1570159X113116660046] [PMCID: PMC3915351] [PMID: ] - DOI - PMC - PubMed
Van Dyck 2007
-
- Van Dyck CH, Tariot PN, Meyers B, Malca Resnick E, Memantine MEM-MD-01 Study Group. A 24-week randomized, controlled trial of memantine in patients with moderate-to-severe Alzheimer disease. Alzheimer Disease & Associated Disorders 2007;21(2):136-43. [DOI: 10.1097/WAD.0b013e318065c495] [PMID: ] - DOI - PubMed
Vannucchi 2014
Van Wijngaarden‐Cremers 2014
-
- Van Wijngaarden-Cremers PJ, Van Eeten E, Groen WB, Van Deurzen PA, Oosterling IJ, Van der Gaag RJ. Gender and age differences in the core triad of impairments in autism spectrum disorders: a systematic review and meta-analysis. Journal of Autism and Developmental Disorders 2014;44(3):627-35. [DOI: 10.1007/s10803-013-1913-9] [PMID: ] - DOI - PubMed
Vasa 2015
Volkmar 2014
Wechsler 2014
-
- Wechsler D. Wechsler Intelligence Scale for Children. 5th edition. Bloomington (MN): Pearson, 2014.
Wei 2012
-
- Wei H, Dobkin C, Sheikh AM, Malik M, Brown WT, Li X. The therapeutic effect of memantine through the stimulation of synapse formation and dendritic spine maturation in autism and fragile X syndrome. PLoS One 2012;7(5):e36981. [DOI: 10.1371/journal.pone.0036981] [PMCID: PMC3352866] [PMID: ] - DOI - PMC - PubMed
WHO 1992
-
- World Health Organization (WHO). The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva (CH): WHO, 1992.
WHO 2018
-
- World Health Organization. International classification of diseases for mortality and morbidity statistics (11th Revision). www.icd.who.int/browse11/l-m/en (accessed 1 December 2020).
Williams 2012
Williams 2013
Williams 2015
Williams 2018
-
- Williams KT. EVT-3: Expressive Vocabulary Test. 3rd edition. San Antonio (TX): Pearson, 2018.