Laboratory-Confirmed COVID-19-Associated Hospitalizations Among Adults During SARS-CoV-2 Omicron BA.2 Variant Predominance - COVID-19-Associated Hospitalization Surveillance Network, 14 States, June 20, 2021-May 31, 2022
- PMID: 36006841
- PMCID: PMC9422959
- DOI: 10.15585/mmwr.mm7134a3
Laboratory-Confirmed COVID-19-Associated Hospitalizations Among Adults During SARS-CoV-2 Omicron BA.2 Variant Predominance - COVID-19-Associated Hospitalization Surveillance Network, 14 States, June 20, 2021-May 31, 2022
Abstract
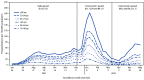
Beginning the week of March 20–26, 2022, the Omicron BA.2 variant of SARS-CoV-2, the virus that causes COVID-19, became the predominant circulating variant in the United States, accounting for >50% of sequenced isolates.* Data from the COVID-19–Associated Hospitalization Surveillance Network (COVID-NET) were analyzed to describe recent COVID-19–associated hospitalization rates among adults aged ≥18 years during the period coinciding with BA.2 predominance (BA.2 period [Omicron BA.2 and BA.2.12.1; March 20–May 31, 2022]). Weekly hospitalization rates (hospitalizations per 100,000 population) among adults aged ≥65 years increased threefold, from 6.9 (week ending April 2, 2022) to 27.6 (week ending May 28, 2022); hospitalization rates in adults aged 18–49 and 50–64 years both increased 1.7-fold during the same time interval. Hospitalization rates among unvaccinated adults were 3.4 times as high as those among vaccinated adults. Among hospitalized nonpregnant patients in this same period, 39.1% had received a primary vaccination series and 1 booster or additional dose; 5.0% had received a primary series and ≥2 boosters or additional doses. All adults should stay up to date† with COVID-19 vaccination, and multiple nonpharmaceutical and medical prevention measures should be used to protect those at high risk for severe COVID-19 illness, irrespective of vaccination status§ (1).
Beginning the week of March 20–26, 2022, the Omicron BA.2 variant of SARS-CoV-2, the virus that causes COVID-19, became the predominant circulating variant in the United States, accounting for >50% of sequenced isolates.* Data from the COVID-19–Associated Hospitalization Surveillance Network (COVID-NET) were analyzed to describe recent COVID-19–associated hospitalization rates among adults aged ≥18 years during the period coinciding with BA.2 predominance (BA.2 period [Omicron BA.2 and BA.2.12.1; March 20–May 31, 2022]). Weekly hospitalization rates (hospitalizations per 100,000 population) among adults aged ≥65 years increased threefold, from 6.9 (week ending April 2, 2022) to 27.6 (week ending May 28, 2022); hospitalization rates in adults aged 18–49 and 50–64 years both increased 1.7-fold during the same time interval. Hospitalization rates among unvaccinated adults were 3.4 times as high as those among vaccinated adults. Among hospitalized nonpregnant patients in this same period, 39.1% had received a primary vaccination series and 1 booster or additional dose; 5.0% had received a primary series and ≥2 boosters or additional doses. All adults should stay up to date† with COVID-19 vaccination, and multiple nonpharmaceutical and medical prevention measures should be used to protect those at high risk for severe COVID-19 illness, irrespective of vaccination status§ (1).
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Andrea George reports grant support from the Council of State and Territorial Epidemiologists (CSTE). Nancy E. Moran reports grant support from CSTE for the population-based Influenza Hospitalization Surveillance Project and COVID-NET activities. Libby Reeg reports institutional support from CSTE. Andy Weigel reports grant support from CSTE for influenza surveillance activities. Evan J. Anderson reports grants for clinical trials from Pfizer, Merck, PaVax, Micron, Sanofi-Pasteur, Janssen, MedImmune, and GlaxoSmithKline; consulting fees from Sanofi-Pasteur, Pfizer, Medscape, Janssen, GlaxoSmithKline, and Moderna; membership on a Kentucky Bioprocessing, Inc., data safety monitoring board and a Sanofi-Pasteur data safety monitoring board, and the endpoint adjudication committee for WCG and ACI Clinical and institutional funding from the National Institutes of Health to conduct clinical trials of COVID-19 vaccines. No other potential conflicts of interest were disclosed.
Figures

References
-
- Delahoy MJ, Whitaker M, O’Halloran A, et al. ; COVID-NET Surveillance Team. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19—COVID-NET, 13 States, March 1–August 22, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1347–54. 10.15585/mmwr.mm6938e1 - DOI - PMC - PubMed
-
- Link-Gelles R, Levy ME, Gaglani M, et al. Effectiveness of 2, 3, and 4 COVID-19 mRNA vaccine doses among immunocompetent adults during periods when SARS-CoV-2 Omicron BA.1 and BA.2/BA.2.12.1 sublineages predominated—VISION Network, 10 states, December 2021–June 2022. MMWR Morb Mortal Wkly Rep 2022;71:931–9. 10.15585/mmwr.mm7129e1 - DOI - PMC - PubMed

