Decarboxylative Sulfinylation Enables a Direct, Metal-Free Access to Sulfoxides from Carboxylic Acids
- PMID: 36006859
- PMCID: PMC9588746
- DOI: 10.1002/anie.202210525
Decarboxylative Sulfinylation Enables a Direct, Metal-Free Access to Sulfoxides from Carboxylic Acids
Abstract
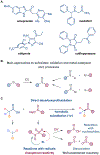
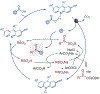
The intermediate oxidation state of sulfoxides is central to the plethora of their applications in chemistry and medicine, yet it presents challenges for an efficient synthetic access, limiting the structural diversity of currently available sulfoxides. Here, we report a data-guided development of direct decarboxylative sulfinylation that enables the previously inaccessible functional group interconversion of carboxylic acids to sulfoxides in a reaction with sulfinates. Given the broad availability of carboxylic acids and the growing synthetic potential of sulfinates, the direct decarboxylative sulfinylation is poised to improve the structural diversity of synthetically accessible sulfoxides. The reaction is facilitated by a kinetically favored sulfoxide formation from the intermediate sulfinyl sulfones, despite the strong thermodynamic preference for the sulfone formation, unveiling the previously unknown and chemoselective radicalophilic sulfinyl sulfone reactivity.
Keywords: Carboxylic Acids; Radical Reactions; Sulfinates; Sulfoxides; Visible Light.
© 2022 Wiley-VCH GmbH.
Figures



Similar articles
-
Photoinduced Radical Sulfinylation of C(sp3)-H Bonds with Sulfinyl Sulfones.Org Lett. 2023 Apr 28;25(16):2883-2888. doi: 10.1021/acs.orglett.3c00868. Epub 2023 Apr 13. Org Lett. 2023. PMID: 37052454
-
Enzymatic Resolution and Decarboxylative Functionalization of α-Sulfinyl Esters.Chemistry. 2024 Feb 1;30(7):e202302996. doi: 10.1002/chem.202302996. Epub 2023 Dec 11. Chemistry. 2024. PMID: 37721804 Free PMC article.
-
Catalytic Decarboxylative Radical Sulfinylation.J Org Chem. 2023 Jun 2;88(11):6671-6681. doi: 10.1021/acs.joc.2c03051. Epub 2023 May 23. J Org Chem. 2023. PMID: 37220021
-
Developments in Synthetic Application of Selenium(IV) Oxide and Organoselenium Compounds as Oxygen Donors and Oxygen-Transfer Agents.Molecules. 2015 Jun 3;20(6):10205-43. doi: 10.3390/molecules200610205. Molecules. 2015. PMID: 26046320 Free PMC article. Review.
-
Ruthenium nanocatalysis on redox reactions.J Nanosci Nanotechnol. 2013 Jul;13(7):4761-86. doi: 10.1166/jnn.2013.7568. J Nanosci Nanotechnol. 2013. PMID: 23901501 Review.
Cited by
-
Decarboxylative Triazolation Enables Direct Construction of Triazoles from Carboxylic Acids.JACS Au. 2023 Feb 16;3(3):813-822. doi: 10.1021/jacsau.2c00606. eCollection 2023 Mar 27. JACS Au. 2023. PMID: 37006773 Free PMC article.
-
Oxysulfonylation of Alkynes with Sodium Sulfinates to Access β-Keto Sulfones Catalyzed by BF3·OEt2.Molecules. 2024 Jul 28;29(15):3559. doi: 10.3390/molecules29153559. Molecules. 2024. PMID: 39124964 Free PMC article.
-
Direct conversion of carboxylic acids to free thiols via radical relay acridine photocatalysis enabled by N-O bond cleavage.Chem Sci. 2023 Dec 5;15(2):644-650. doi: 10.1039/d3sc05513b. eCollection 2024 Jan 3. Chem Sci. 2023. PMID: 38179514 Free PMC article.
-
Multimodal Acridine Photocatalysis Enables Direct Access to Thiols from Carboxylic Acids and Elemental Sulfur.ACS Catal. 2024 May 3;14(9):6973-6980. doi: 10.1021/acscatal.4c01289. Epub 2024 Apr 19. ACS Catal. 2024. PMID: 38737399 Free PMC article.
-
Acridine photocatalysis enables tricomponent direct decarboxylative amine construction.Chem Sci. 2024 May 22;15(25):9582-9590. doi: 10.1039/d4sc02356k. eCollection 2024 Jun 26. Chem Sci. 2024. PMID: 38939159 Free PMC article.
References
-
- Oae S, Organic Sulfur Chemistry: Structure and Mechanism, CRC Press, Boca Raton, 1991;
- Patai S, Rapport Z, Eds., The Chemistry of Sulfonic Acids, Esters and their Derivatives, John Wiley & Sons, New York, 1991;
- Lee C-F, Basha RS, Badsara SS, Top. Curr. Chem 2018, 376, 25; - PubMed
- Kaiser D, Klose I, Oost R, Neuhaus J, Maulide N, Chem. Rev 2019, 119, 8701–8780; - PMC - PubMed
- Zhou M, Tsien J, Qin T, Angew. Chem. Int. Ed 2020, 59, 7372–7376; - PubMed
- Angew. Chem 2020, 132, 7442–7446;
- Hervieu C, Kirillova MS, Suárez T, Müller M, Merino E, Nevado C, Nat. Chem 2021, 13, 327–334; - PubMed
- Zhang Z-X, Willis MC, Chem 2022, 8, 1137–1146.
-
- Patani GA, LaVoie EJ, Chem. Rev 1996, 96, 3147–3176; - PubMed
- Brown N, Ed., Bioisosteres in Medicinal Chemistry, Wiley-VCH, Weinheim, 2012.
-
- Patai S, Ed., The Chemistry of Sulfinic Acids, Esters and their Derivatives, John Wiley & Sons, Ltd, New Jersey, 1990;
- Fernandez I, Khiar N, Chem. Rev 2003, 103, 3651–3706; - PubMed
- Feldman KS, Fodor MD, J. Am. Chem. Soc 2008, 130, 14964–14965; - PubMed
- Kaldre D, Klose I, Maulide N, Science 2018, 361, 664–667; - PubMed
- Yan J, Pulis AP, Perry GJP, Procter DJ, Angew. Chem., Int. Ed 2019, 58, 15675–15679; - PubMed
- Angew. Chem 2019, 131, 15822–15826; - PubMed
- Leypold M, D’Angelo KA, Movassaghi M, Org. Lett 2020, 22, 8802–8807; - PMC - PubMed
- Li G, Nieves-Quinones Y, Zhang H, Liang Q, Su S, Liu Q, Kozlowski MC, Jia T. Nat. Commun 2020, 11, 2890. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

