Universal protection against influenza viruses by multi-subtype neuraminidase and M2 ectodomain virus-like particle
- PMID: 36006890
- PMCID: PMC9409530
- DOI: 10.1371/journal.ppat.1010755
Universal protection against influenza viruses by multi-subtype neuraminidase and M2 ectodomain virus-like particle
Abstract
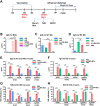
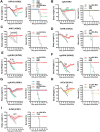
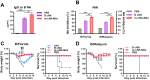
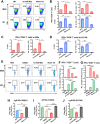
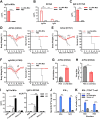
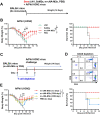
Annual influenza vaccination is recommended to update the variable hemagglutinin antigens. Here, we first designed a virus-like particle (VLP) displaying consensus multi-neuraminidase (NA) subtypes (cN1, cN2, B cNA) and M2 ectodomain (M2e) tandem repeat (m-cNA-M2e VLP). Vaccination of mice with m-cNA-M2e VLP induced broad NA inhibition (NAI), and M2e antibodies as well as interferon-gamma secreting T cell responses. Mice vaccinated with m-cNA-M2e VLP were protected against influenza A (H1N1, H5N1, H3N2, H9N2, H7N9) and influenza B (Yamagata and Victoria lineage) viruses containing substantial antigenic variations. Protective immune contributors include cellular and humoral immunity as well as antibody-dependent cellular cytotoxicity. Furthermore, comparable cross protection by m-cNA-M2e VLP vaccination was induced in aged mice. This study supports a novel strategy of developing a universal vaccine against influenza A and B viruses potentially in both young and aged populations by inducing multi-NA subtype and M2e immunity with a single VLP entity.
Conflict of interest statement
The authors declare no conflicts of competing interest exits.
Figures


Similar articles
-
Supplementation of seasonal vaccine with multi-subtype neuraminidase and M2 ectodomain virus-like particle improves protection against homologous and heterologous influenza viruses in aged mice.Antiviral Res. 2024 May;225:105877. doi: 10.1016/j.antiviral.2024.105877. Epub 2024 Mar 30. Antiviral Res. 2024. PMID: 38561077 Free PMC article.
-
Cross-protection against influenza viruses by chimeric M2e-H3 stalk protein or multi-subtype neuraminidase plus M2e virus-like particle vaccine in ferrets.Virology. 2024 Jul;595:110097. doi: 10.1016/j.virol.2024.110097. Epub 2024 Apr 25. Virology. 2024. PMID: 38685171 Free PMC article.
-
Chimeric virus-like particles of nodavirus displaying M2e of human and avian influenza A viruses as a potential dual-use vaccine: Inducing a broader immune response and protecting mice against viral infections.Vaccine. 2025 May 22;56:127165. doi: 10.1016/j.vaccine.2025.127165. Epub 2025 Apr 25. Vaccine. 2025. PMID: 40286563
-
Cross protection by inactivated recombinant influenza viruses containing chimeric hemagglutinin conjugates with a conserved neuraminidase or M2 ectodomain epitope.Virology. 2020 Nov;550:51-60. doi: 10.1016/j.virol.2020.08.003. Epub 2020 Aug 22. Virology. 2020. PMID: 32882637 Free PMC article.
-
Influenza A viruses: why focusing on M2e-based universal vaccines.Virus Genes. 2011 Feb;42(1):1-8. doi: 10.1007/s11262-010-0547-7. Epub 2010 Nov 17. Virus Genes. 2011. PMID: 21082230 Review.
Cited by
-
Capsid virus-like particle display improves recombinant influenza neuraminidase antigen stability and immunogenicity in mice.iScience. 2024 May 20;27(6):110038. doi: 10.1016/j.isci.2024.110038. eCollection 2024 Jun 21. iScience. 2024. PMID: 38883830 Free PMC article.
-
Delivery of dendritic cells targeting 3M2e-HA2 nanoparticles with a CpG adjuvant via lysosomal escape of Salmonella enhances protection against H9N2 avian influenza virus.Poult Sci. 2025 Jan;104(1):104616. doi: 10.1016/j.psj.2024.104616. Epub 2024 Dec 1. Poult Sci. 2025. PMID: 39631272 Free PMC article.
-
CircRNA based multivalent neuraminidase vaccine induces broad protection against influenza viruses in mice.NPJ Vaccines. 2024 Sep 16;9(1):170. doi: 10.1038/s41541-024-00963-4. NPJ Vaccines. 2024. PMID: 39285168 Free PMC article.
-
Clade 2.3.4.4b highly pathogenic avian influenza H5N1 viruses: knowns, unknowns, and challenges.J Virol. 2025 Jun 17;99(6):e0042425. doi: 10.1128/jvi.00424-25. Epub 2025 May 9. J Virol. 2025. PMID: 40340397 Free PMC article. Review.
-
Supplementation of seasonal vaccine with multi-subtype neuraminidase and M2 ectodomain virus-like particle improves protection against homologous and heterologous influenza viruses in aged mice.Antiviral Res. 2024 May;225:105877. doi: 10.1016/j.antiviral.2024.105877. Epub 2024 Mar 30. Antiviral Res. 2024. PMID: 38561077 Free PMC article.
References
-
- CDC. Seasonal Influenza Vaccine Effectiveness, 2004–2019. https://wwwcdcgov/flu/vaccines-work/effectiveness-studieshtm#figure.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

