The baseline immunological and hygienic status of pigs impact disease severity of African swine fever
- PMID: 36006954
- PMCID: PMC9409533
- DOI: 10.1371/journal.ppat.1010522
The baseline immunological and hygienic status of pigs impact disease severity of African swine fever
Abstract
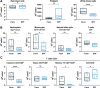
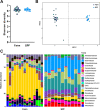
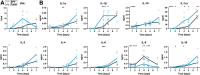
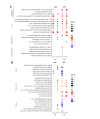
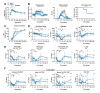
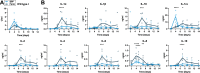
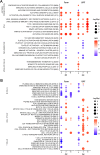
African Swine Fever virus (ASFV) is a large double-enveloped DNA virus of the Asfarviridae family that causes a lethal hemorrhagic disease in domestic pigs and wild boars. Since 2007, a highly virulent genotype II strain has emerged and spread in Europe and South-East Asia, where millions of animals succumbed to the disease. Field- and laboratory-attenuated strains of ASFV cause highly variable clinical disease severity and survival, and mechanisms remain unclear. We hypothesized that the immunological and hygienic status of pigs is a determinant of ASF disease course. Here we compared the immunological profile at baseline and in response to ASFV infection in specific pathogen-free (SPF) and farm-raised Large White domestic pigs. At steady state, SPF pigs showed lower white blood cell counts and a lower basal inflammatory and antiviral transcriptomic profile compared to farm pigs, associated with profound differences in gut microbiome composition. After inoculation with a highly virulent ASFV genotype II strain (Armenia 2008), severe clinical signs, viremia and pro-inflammatory cytokines appeared sooner in SPF pigs, indicating a reduced capacity to control early virus replication. In contrast, during infection with an attenuated field isolate (Estonia 2014), SPF pigs presented a milder and shorter clinical disease with full recovery, whereas farm pigs presented severe protracted disease with 50% lethality. Interestingly, farm pigs showed higher production of inflammatory cytokines, whereas SPF pigs produced more anti-inflammatory IL-1ra early after infection and presented a stronger expansion of leukocytes in the recovery phase. Altogether, our data indicate that the hygiene-dependent innate immune status has a double-edge sword impact on immune responses in ASF pathogenesis. While the higher baseline innate immune activity helps the host in reducing initial virus replication, it promotes immunopathological cytokine responses, and delays lymphocyte proliferation after infection with an attenuated strain. Such effects should be considered for live vaccine development and vigilance.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Transcriptome profile of spleen tissues from locally-adapted Kenyan pigs (Sus scrofa) experimentally infected with three varying doses of a highly virulent African swine fever virus genotype IX isolate: Ken12/busia.1 (ken-1033).BMC Genomics. 2022 Jul 19;23(1):522. doi: 10.1186/s12864-022-08754-8. BMC Genomics. 2022. PMID: 35854219 Free PMC article.
-
Comparative evaluation of disease dynamics in wild boar and domestic pigs experimentally inoculated intranasally with the European highly virulent African swine fever virus genotype II strain "Armenia 2007".Vet Res. 2024 Jul 15;55(1):89. doi: 10.1186/s13567-024-01343-5. Vet Res. 2024. PMID: 39010163 Free PMC article.
-
Deletion of E184L, a Putative DIVA Target from the Pandemic Strain of African Swine Fever Virus, Produces a Reduction in Virulence and Protection against Virulent Challenge.J Virol. 2022 Jan 12;96(1):e0141921. doi: 10.1128/JVI.01419-21. Epub 2021 Oct 20. J Virol. 2022. PMID: 34668772 Free PMC article.
-
[African swine fever in Russian Federation].Vopr Virusol. 2012 Sep-Oct;57(5):4-10. Vopr Virusol. 2012. PMID: 23248852 Review. Russian.
-
Regulation of antiviral immune response by African swine fever virus (ASFV).Virol Sin. 2022 Apr;37(2):157-167. doi: 10.1016/j.virs.2022.03.006. Epub 2022 Mar 9. Virol Sin. 2022. PMID: 35278697 Free PMC article. Review.
Cited by
-
Disease Course of Korean African Swine Fever Virus in Domestic Pigs Exposed Intraorally, Intranasally, Intramuscularly, and by Direct Contact with Infected Pigs.Viruses. 2024 Mar 11;16(3):433. doi: 10.3390/v16030433. Viruses. 2024. PMID: 38543798 Free PMC article.
-
Transcriptome profiles of organ tissues from pigs experimentally infected with African swine fever virus in early phase of infection.Emerg Microbes Infect. 2024 Dec;13(1):2366406. doi: 10.1080/22221751.2024.2366406. Epub 2024 Jun 26. Emerg Microbes Infect. 2024. PMID: 38847223 Free PMC article.
-
Replication Characteristics of African Swine Fever Virus (ASFV) Genotype I E70 and ASFV Genotype II Belgium 2018/1 in Perivenous Macrophages Using Established Vein Explant Model.Viruses. 2024 Oct 12;16(10):1602. doi: 10.3390/v16101602. Viruses. 2024. PMID: 39459935 Free PMC article.
-
African Swine Fever Virus Infection and Cytokine Response In Vivo: An Update.Viruses. 2023 Jan 14;15(1):233. doi: 10.3390/v15010233. Viruses. 2023. PMID: 36680273 Free PMC article. Review.
-
2023 International African Swine Fever Workshop: Critical Issues That Need to Be Addressed for ASF Control.Viruses. 2023 Dec 19;16(1):4. doi: 10.3390/v16010004. Viruses. 2023. PMID: 38275939 Free PMC article.
References
-
- Montgomery RE. On A Form of Swine Fever Occurring in British East Africa (Kenya Colony). Journal of Comparative Pathology and Therapeutics. 1921;34:159–91. doi: 10.1016/S0368-1742(21)80031-4 EUSTACEMONTGOMERY1921159. - DOI

