Pathogenomics and clinical recurrence influence biofilm capacity of Escherichia coli isolated from canine urinary tract infections
- PMID: 36006972
- PMCID: PMC9409522
- DOI: 10.1371/journal.pone.0270461
Pathogenomics and clinical recurrence influence biofilm capacity of Escherichia coli isolated from canine urinary tract infections
Abstract
Biofilm formation enhances bacteria's ability to colonize unique niches while protecting themselves from environmental stressors. Escherichia coli that colonize the urinary tract can protect themselves from the harsh bladder environment by forming biofilms. These biofilms promote persistence that can lead to chronic and recurrent urinary tract infections (UTI). While biofilm formation is frequently studied among urinary E. coli, its association with other pathogenic mechanisms and adaptations in certain host populations remains poorly understood. Here we utilized whole genome sequencing and retrospective medical record analysis to investigate associations between the population structure, phenotypic resistance, resistome, virulome, and patient demographic and clinical findings of 104 unique urinary E. coli and their capacity to form biofilms. We show that population structure including multilocus sequence typing and Clermont phylogrouping had no association with biofilm capacity. Among clinical factors, exposure to multiple antibiotics within that past 30 days and a clinical history of recurrent UTIs were positively associated with biofilm formation. In contrast, phenotypic antimicrobial reduced susceptibility and corresponding acquired resistance genes were negatively associated with biofilm formation. While biofilm formation was associated with increased virulence genes within the cumulative virulome, individual virulence genes did not influence biofilm capacity. We identified unique virulotypes among different strata of biofilm formation and associated the presence of the tosA/R-ibeA gene combination with moderate to strong biofilm formation. Our findings suggest that E. coli causing UTI in dogs utilize a heterogenous mixture of virulence genes to reach a biofilm phenotype, some of which may promote robust biofilm capacity. Antimicrobial use may select for two populations, non-biofilm formers that maintain an arsenal of antimicrobial resistance genes to nullify treatment and a second that forms durable biofilms to avoid therapeutic insults.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
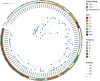
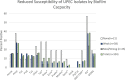

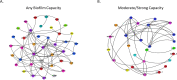

Similar articles
-
Previous Antibiotic Exposure Reshapes the Population Structure of Infecting Uropathogenic Escherichia coli Strains by Selecting for Antibiotic Resistance over Urovirulence.Microbiol Spectr. 2023 Aug 17;11(4):e0524222. doi: 10.1128/spectrum.05242-22. Epub 2023 Jun 20. Microbiol Spectr. 2023. PMID: 37338386 Free PMC article.
-
Bacterial characteristics of importance for recurrent urinary tract infections caused by Escherichia coli.Dan Med Bull. 2011 Apr;58(4):B4187. Dan Med Bull. 2011. PMID: 21466767 Review.
-
Comprehensive phenotypic and genotypic characterization and comparison of virulence, biofilm, and antimicrobial resistance in urinary Escherichia coli isolated from canines.Vet Microbiol. 2020 Oct;249:108822. doi: 10.1016/j.vetmic.2020.108822. Epub 2020 Aug 13. Vet Microbiol. 2020. PMID: 32937249
-
Characteristics of Dogs with Biofilm-Forming Escherichia Coli Urinary Tract Infections.J Vet Intern Med. 2018 Sep;32(5):1645-1651. doi: 10.1111/jvim.15231. Epub 2018 Aug 7. J Vet Intern Med. 2018. PMID: 30084122 Free PMC article.
-
A systematic review and meta-analysis of antibiotic resistance patterns, and the correlation between biofilm formation with virulence factors in uropathogenic E. coli isolated from urinary tract infections.Microb Pathog. 2020 Jul;144:104196. doi: 10.1016/j.micpath.2020.104196. Epub 2020 Apr 10. Microb Pathog. 2020. PMID: 32283258
Cited by
-
Patterns of Drug Resistance and Bacterial Pathogen Distribution in Patients with Urinary Tract Infections in the Jiaxing Region from 2020 to 2022.Infect Drug Resist. 2023 Sep 6;16:5911-5921. doi: 10.2147/IDR.S424158. eCollection 2023. Infect Drug Resist. 2023. PMID: 37700799 Free PMC article.
-
Evaluation of antibiotic resistance, toxin-antitoxin systems, virulence factors, biofilm-forming strength and genetic linkage of Escherichia coli strains isolated from bloodstream infections of leukemia patients.BMC Microbiol. 2023 Nov 4;23(1):327. doi: 10.1186/s12866-023-03081-8. BMC Microbiol. 2023. PMID: 37925405 Free PMC article.
-
Antimicrobial Resistance and Biofilm-Forming Ability in ESBL-Producing and Non-ESBL-Producing Escherichia coli and Klebsiella pneumoniae Isolated from Canine Urinary Samples from Italy.Antibiotics (Basel). 2025 Jan 3;14(1):31. doi: 10.3390/antibiotics14010031. Antibiotics (Basel). 2025. PMID: 39858317 Free PMC article.
-
Analysis of phylogroups, biofilm formation, virulence factors, antibiotic resistance and molecular typing of uropathogenic Escherichia coli strains isolated from patients with recurrent and non-recurrent urinary tract infections.BMC Infect Dis. 2025 Feb 24;25(1):267. doi: 10.1186/s12879-025-10635-w. BMC Infect Dis. 2025. PMID: 39994590 Free PMC article.
-
Previous Antibiotic Exposure Reshapes the Population Structure of Infecting Uropathogenic Escherichia coli Strains by Selecting for Antibiotic Resistance over Urovirulence.Microbiol Spectr. 2023 Aug 17;11(4):e0524222. doi: 10.1128/spectrum.05242-22. Epub 2023 Jun 20. Microbiol Spectr. 2023. PMID: 37338386 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

