Tamoxifen-resistant breast cancer cells exhibit reactivity with Wisteria floribunda agglutinin
- PMID: 36006984
- PMCID: PMC9409572
- DOI: 10.1371/journal.pone.0273513
Tamoxifen-resistant breast cancer cells exhibit reactivity with Wisteria floribunda agglutinin
Abstract
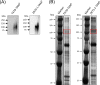
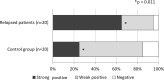
Glycosylation is one of the most important post-translational modifications of cell surface proteins involved in the proliferation, metastasis and treatment resistance of cancer cells. However, little is known about the role of glycosylation as the mechanism of breast cancer cell resistance to endocrine therapy. Herein, we aimed to identify the glycan profiles of tamoxifen-resistant human breast cancer cells, and their potential as predictive biomarkers for endocrine therapy. We established tamoxifen-resistant cells from estrogen receptor-positive human breast cancer cell lines, and their membrane-associated proteins were subjected to lectin microarray analysis. To confirm differential lectin binding to cellular glycoproteins, we performed lectin blotting analyses after electrophoretic separation of the glycoproteins. Mass spectrometry of the tryptic peptides of the lectin-bound glycoproteins was further conducted to identify glycoproteins binding to the above lectins. Finally, expression of the glycans that were recognized by a lectin was investigated using clinical samples from patients who received tamoxifen treatment after curative surgery. Lectin microarray analysis revealed that the membrane fractions of tamoxifen-resistant breast cancer cells showed increased binding to Wisteria floribunda agglutinin (WFA) compared to tamoxifen-sensitive cells. Glycoproteins seemed to be responsible for the differential WFA binding and the results of mass spectrometry revealed several membrane glycoproteins, such as CD166 and integrin beta-1, as candidates contributing to increased WFA binding. In clinical samples, strong WFA staining was more frequently observed in patients who had developed distant metastasis during tamoxifen treatment compared with non-relapsed patients. Therefore, glycans recognized by WFA are potentially useful as predictive markers to identify the tamoxifen-resistant and relapse-prone subset of estrogen receptor-positive breast cancer patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
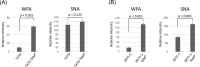

Similar articles
-
Wisteria floribunda agglutinin-binding glycan expression is decreased in endometriomata.Reprod Biol Endocrinol. 2014 Oct 24;12:100. doi: 10.1186/1477-7827-12-100. Reprod Biol Endocrinol. 2014. PMID: 25344456 Free PMC article.
-
Wisteria floribunda Agglutinin and Its Reactive-Glycan-Carrying Prostate-Specific Antigen as a Novel Diagnostic and Prognostic Marker of Prostate Cancer.Int J Mol Sci. 2017 Jan 26;18(2):261. doi: 10.3390/ijms18020261. Int J Mol Sci. 2017. PMID: 28134773 Free PMC article.
-
Wisteria floribunda agglutinin staining for the quantitative assessment of cardiac fibrogenic activity in a mouse model of dilated cardiomyopathy.Lab Invest. 2019 Nov;99(11):1749-1765. doi: 10.1038/s41374-019-0279-9. Epub 2019 Jun 28. Lab Invest. 2019. PMID: 31253865
-
Wisteria floribunda agglutinin positive glycobiomarkers: a unique lectin as a serum biomarker probe in various diseases.Expert Rev Proteomics. 2018 Feb;15(2):183-190. doi: 10.1080/14789450.2018.1419066. Epub 2017 Dec 21. Expert Rev Proteomics. 2018. PMID: 29265940 Review.
-
Lectins as a promising therapeutic agent for breast cancer: A review.Breast Dis. 2024;43(1):193-211. doi: 10.3233/BD-230047. Breast Dis. 2024. PMID: 38905027 Free PMC article. Review.
Cited by
-
Lectin: A Molecular Tool in Cancer Diagnosis and Therapy with Special Reference to Reproductive Cancers.Mol Biotechnol. 2025 Feb;67(2):456-468. doi: 10.1007/s12033-024-01086-w. Epub 2024 Mar 8. Mol Biotechnol. 2025. PMID: 38456960 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

