Outcomes in Patients with Poor-Risk Cytogenetics with or without TP53 Mutations Treated with Venetoclax and Azacitidine
- PMID: 36007102
- PMCID: PMC9751752
- DOI: 10.1158/1078-0432.CCR-22-1183
Outcomes in Patients with Poor-Risk Cytogenetics with or without TP53 Mutations Treated with Venetoclax and Azacitidine
Abstract
Purpose: To evaluate efficacy and safety of venetoclax + azacitidine in treatment-naïve patients with acute myeloid leukemia harboring poor-risk cytogenetics and TP53mut or TP53wt.
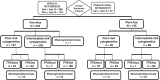
Patients and methods: We analyzed data from a phase III study (NCT02993523) comparing venetoclax (400 mg orally days 1-28) + azacitidine (75 mg/m2 days 1-7) or placebo + azacitidine, and from a phase Ib study (NCT02203773) of venetoclax + azacitidine. Patients were ineligible for intensive therapy. TP53 status was analyzed centrally; cytogenetic studies were performed locally.
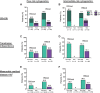
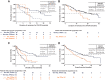
Results: Patients (n = 127) with poor-risk cytogenetics receiving venetoclax + azacitidine (TP53wt = 50; TP53mut = 54) were compared with patients with poor-risk cytogenetics (n = 56) receiving azacitidine alone (TP53wt = 22; TP53mut = 18).For poor-risk cytogenetics + TP53wt patients, venetoclax + azacitidine versus azacitidine alone resulted in composite remission rates (CRc) of 70% versus 23%, median duration of remission (DoR) of 18.4 versus 8.5 months, and median overall survival (OS) of 23.4 versus 11.3 months, respectively. Outcomes with venetoclax + azacitidine were comparable with similarly treated patients with intermediate-risk cytogenetics and TP53wt.For poor-risk cytogenetics + TP53mut patients, venetoclax + azacitidine versus azacitidine alone resulted in CRc of 41% versus 17%, median DoR of 6.5 versus 6.7 months, and median OS of 5.2 versus 4.9 months, respectively.For poor-risk cytogenetics + TP53mut patients, predominant grade ≥3 adverse events (AE) for venetoclax + azacitidine versus azacitidine were febrile neutropenia (55%/39%), thrombocytopenia (28%/28%), neutropenia (26%/17%), anemia (13%/6%), and pneumonia (28%/33%). AEs were comparable between TP53mut and TP53wt patients.
Conclusions: In poor-risk cytogenetics + TP53mut patients, venetoclax + azacitidine improved remission rates but not DoR or OS compared with azacitidine alone. However, in poor-risk cytogenetics + TP53wt patients, venetoclax + azacitidine resulted in higher remission rates and longer DoR and OS than azacitidine alone, with outcomes comparable with similarly treated patients with intermediate-risk cytogenetics. Toxicities were similar in TP53mut and TP53wt patients. See related commentary by Green and Zeidner, p. 5235.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures

Comment in
-
TP53 or Not TP53: That Is the Question.Clin Cancer Res. 2022 Dec 15;28(24):5235-5237. doi: 10.1158/1078-0432.CCR-22-2664. Clin Cancer Res. 2022. PMID: 36197410
References
-
- Rücker FG, Schlenk RF, Bullinger L, Kayser S, Teleanu V, Kett H, et al. . TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood 2012;119:2114–21. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

