Ion channels and channelopathies in glomeruli
- PMID: 36007181
- PMCID: PMC9662803
- DOI: 10.1152/physrev.00013.2022
Ion channels and channelopathies in glomeruli
Abstract
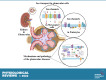
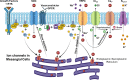
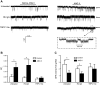
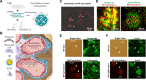
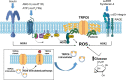
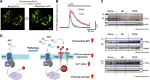
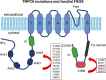
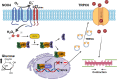
An essential step in renal function entails the formation of an ultrafiltrate that is delivered to the renal tubules for subsequent processing. This process, known as glomerular filtration, is controlled by intrinsic regulatory systems and by paracrine, neuronal, and endocrine signals that converge onto glomerular cells. In addition, the characteristics of glomerular fluid flow, such as the glomerular filtration rate and the glomerular filtration fraction, play an important role in determining blood flow to the rest of the kidney. Consequently, disease processes that initially affect glomeruli are the most likely to lead to end-stage kidney failure. The cells that comprise the glomerular filter, especially podocytes and mesangial cells, express many different types of ion channels that regulate intrinsic aspects of cell function and cellular responses to the local environment, such as changes in glomerular capillary pressure. Dysregulation of glomerular ion channels, such as changes in TRPC6, can lead to devastating glomerular diseases, and a number of channels, including TRPC6, TRPC5, and various ionotropic receptors, are promising targets for drug development. This review discusses glomerular structure and glomerular disease processes. It also describes the types of plasma membrane ion channels that have been identified in glomerular cells, the physiological and pathophysiological contexts in which they operate, and the pathways by which they are regulated and dysregulated. The contributions of these channels to glomerular disease processes, such as focal segmental glomerulosclerosis (FSGS) and diabetic nephropathy, as well as the development of drugs that target these channels are also discussed.
Keywords: KCa1.1 channels; NMDA receptors; TRPC channels; focal segmental glomerulosclerosis; glomerular filtration rate; store-operated calcium channels.
Conflict of interest statement
S.E.D. has received research contracts from Pfizer Inc. and Walden Biosciences Inc. He has received speakers honoraria from Amgen Inc., Walden Bioscience Inc., and Aredelyx Inc., and serves on the scientific advisory board of Actio Biosciences Inc. No other authors have any conflicts of interest, financial or otherwise, to disclose.
Figures






Similar articles
-
TRPC Channels in Proteinuric Kidney Diseases.Cells. 2019 Dec 23;9(1):44. doi: 10.3390/cells9010044. Cells. 2019. PMID: 31877991 Free PMC article. Review.
-
TRPC channels: Regulation, dysregulation and contributions to chronic kidney disease.Biochim Biophys Acta Mol Basis Dis. 2019 Jun 1;1865(6):1041-1066. doi: 10.1016/j.bbadis.2019.04.001. Epub 2019 Apr 4. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 30953689 Review.
-
TRPC6 channel as an emerging determinant of the podocyte injury susceptibility in kidney diseases.Am J Physiol Renal Physiol. 2015 Sep 1;309(5):F393-7. doi: 10.1152/ajprenal.00186.2015. Epub 2015 Jun 17. Am J Physiol Renal Physiol. 2015. PMID: 26084930 Free PMC article. Review.
-
Regulation of TRPC6 ion channels in podocytes - Implications for focal segmental glomerulosclerosis and acquired forms of proteinuric diseases.Acta Physiol Hung. 2015 Sep;102(3):241-51. doi: 10.1556/036.102.2015.3.2. Acta Physiol Hung. 2015. PMID: 26551740 Review.
-
TRPC channel modulation in podocytes-inching toward novel treatments for glomerular disease.Pediatr Nephrol. 2011 Jul;26(7):1057-64. doi: 10.1007/s00467-010-1718-4. Epub 2010 Dec 16. Pediatr Nephrol. 2011. PMID: 21161284 Free PMC article. Review.
Cited by
-
Integrating genome-wide association studies and transcriptomics prioritizes drug targets for meningioma.Brain Commun. 2025 Feb 5;7(2):fcaf053. doi: 10.1093/braincomms/fcaf053. eCollection 2025. Brain Commun. 2025. PMID: 40046334 Free PMC article.
-
Mitochondrial metabolic reprogramming in diabetic kidney disease.Cell Death Dis. 2024 Jun 24;15(6):442. doi: 10.1038/s41419-024-06833-0. Cell Death Dis. 2024. PMID: 38910210 Free PMC article. Review.
-
Renal Implications of Kappa Opioid Receptor Signaling in Sprague-Dawley Rats.Function (Oxf). 2025 Aug 1;6(4):zqaf028. doi: 10.1093/function/zqaf028. Function (Oxf). 2025. PMID: 40586679 Free PMC article.
-
The Effects of TRPC6 Knockout in Animal Models of Kidney Disease.Biomolecules. 2022 Nov 18;12(11):1710. doi: 10.3390/biom12111710. Biomolecules. 2022. PMID: 36421724 Free PMC article. Review.
-
The small GTPase regulatory protein Rac1 drives podocyte injury independent of cationic channel protein TRPC5.Kidney Int. 2023 Jun;103(6):1056-1062. doi: 10.1016/j.kint.2023.01.016. Epub 2023 Feb 5. Kidney Int. 2023. PMID: 36750145 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

