Effect of a Multistrain Probiotic on Leaky Gut in Patients with Diarrhea-Predominant Irritable Bowel Syndrome: A Pilot Study
- PMID: 36007493
- PMCID: PMC10906476
- DOI: 10.1159/000526712
Effect of a Multistrain Probiotic on Leaky Gut in Patients with Diarrhea-Predominant Irritable Bowel Syndrome: A Pilot Study
Abstract
Background: A probiotic mixture prevented epithelial barrier impairment in various experimental models. The objective was to evaluate its effects in patients suffering from IBS with diarrhea (IBS-D) with confirmed leaky gut.
Methods: IBS-D patients with increased intestinal permeability measured by radionuclide tracers were enrolled in this pilot, open-label, prospective, interventional, single-center, Phase IV study. Patients received two capsules of a multistrain probiotic a day for 30 days and were evaluated by repeated intestinal permeability tests, the Bristol Stool Scale, and patient-perceived quality of life and satisfaction.
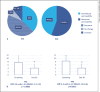
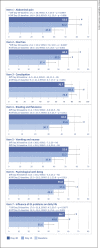
Results: Of the 30 enrolled patients (mean age: 42.1 [SD: 13.1] years; female: 60%), 27 completed the study (full analysis set [FAS]), and 18 had no major protocol violation (per protocol set [PPS]). On D30, an improvement of intestinal permeability was observed in 81.5% of patients in FAS, normalization being observed in 37% of the participants (44% in PPS). The mean intestinal permeability was significantly decreased: baseline minus D30, 3.4 (95% CI: 1.7, 5.2); the IBS-QOL total score was significantly increased: D30 minus baseline, 8.0 (95% CI: 3.0, 12.9); and stool consistency was significantly improved. On D15 and D30, 96.3% of patients claimed that their IBS symptoms had been satisfactory alleviated, and a significant improvement was reported for the following VAS-IBS items: abdominal pain, diarrhea, and impact of gastrointestinal problems in daily life. Compliance and tolerance were satisfactory.
Conclusion: The multistrain probiotic tested may reduce intestinal permeability in a considerable proportion of patients and may improve abdominal pain, stool consistency, and quality of life. These results pave the way for larger, placebo-controlled clinical studies.
Keywords: Intestinal permeability; Irritable bowel syndrome; Leaky gut syndrome; Probiotics; Quality of life.
© 2022 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Samira Ait Abdellah and Caroline Gal are employees of PiLeJe Laboratoire. Lucrezia Laterza is a lecturer for Janssen and a consultant for Actial Farmaceutica. Antonio Gasbarrini is an associate director of the journal. Other authors declare no conflict of interest.
Figures
Similar articles
-
A randomized placebo-controlled clinical trial of a multi-strain probiotic formulation (Bio-Kult®) in the management of diarrhea-predominant irritable bowel syndrome.BMC Gastroenterol. 2018 May 25;18(1):71. doi: 10.1186/s12876-018-0788-9. BMC Gastroenterol. 2018. PMID: 29801486 Free PMC article. Clinical Trial.
-
A randomized double-blind, placebo-controlled trial to evaluate the safety and efficacy of live Bifidobacterium longum CECT 7347 (ES1) and heat-treated Bifidobacterium longum CECT 7347 (HT-ES1) in participants with diarrhea-predominant irritable bowel syndrome.Gut Microbes. 2024 Jan-Dec;16(1):2338322. doi: 10.1080/19490976.2024.2338322. Epub 2024 Apr 17. Gut Microbes. 2024. PMID: 38630015 Free PMC article. Clinical Trial.
-
Multistrain Probiotics Plus Vitamin D Improve Gut Barrier Function and Gut Microbiota Composition in Irritable Bowel Syndrome Without Constipation: Results from a Double-Blind, Randomized, Placebo-Controlled Trial.Nutrients. 2025 May 18;17(10):1708. doi: 10.3390/nu17101708. Nutrients. 2025. PMID: 40431448 Free PMC article. Clinical Trial.
-
Emerging role of probiotics and antimicrobials in the management of irritable bowel syndrome.Curr Med Res Opin. 2014 Jul;30(7):1405-15. doi: 10.1185/03007995.2014.908278. Epub 2014 Apr 14. Curr Med Res Opin. 2014. PMID: 24666019 Review.
-
Effectiveness of Probiotic Use in Alleviating Symptoms of Irritable Bowel Syndrome: A Systematic Review.Cureus. 2024 Apr 15;16(4):e58306. doi: 10.7759/cureus.58306. eCollection 2024 Apr. Cureus. 2024. PMID: 38752062 Free PMC article. Review.
Cited by
-
Probiotics and Prebiotics for the Treatment of Irritable Bowel Syndrome-A Narrative Review.J Clin Med. 2024 Oct 23;13(21):6337. doi: 10.3390/jcm13216337. J Clin Med. 2024. PMID: 39518476 Free PMC article. Review.
-
Depression in Diarrhea-Predominant IBS Patients: Exploring the Link Between Gut Barrier Dysfunction and Erythrocyte Polyunsaturated Fatty Acid Levels.J Clin Med. 2025 Apr 5;14(7):2483. doi: 10.3390/jcm14072483. J Clin Med. 2025. PMID: 40217932 Free PMC article.
-
Exploring the dark side of probiotics to pursue light: Intrinsic and extrinsic risks to be opportunistic pathogens.Curr Res Food Sci. 2025 Mar 31;10:101044. doi: 10.1016/j.crfs.2025.101044. eCollection 2025. Curr Res Food Sci. 2025. PMID: 40235735 Free PMC article. Review.
-
Intestinal Barrier Impairment, Preservation, and Repair: An Update.Nutrients. 2024 Oct 15;16(20):3494. doi: 10.3390/nu16203494. Nutrients. 2024. PMID: 39458489 Free PMC article. Review.
-
A Mini Literature Review of Probiotics: Transforming Gastrointestinal Health Through Evidence-Based Insights.Cureus. 2024 Mar 27;16(3):e57055. doi: 10.7759/cureus.57055. eCollection 2024 Mar. Cureus. 2024. PMID: 38681263 Free PMC article. Review.
References
-
- Oka P, Parr H, Barberio B, Black CJ, Savarino EV, Ford AC. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5((10)):908–917. - PubMed
-
- Barbara G, Zecchi L, Barbaro R, Cremon C, Bellacosa L, Marcellini M, et al. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J Clin Gastroenterol. 2012;46((Suppl l)):S52–S55. - PubMed
-
- Quigley EMM, Fried M, Gwee K-A, Khalif I, Hungin P, Lindberg G, et al. Irritable bowel syndrome: a global perspective 2015. Available from: https://www.worldgastroenterology.org/UserFiles/file/guidelines/irritabl.... - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

