Ferric Citrate Hydrate Has Little Impact on Hyperplasia of Enterochromaffin Cells in the Rat Small Intestine Compared to Sodium Ferrous Citrate
- PMID: 36007495
- PMCID: PMC9838135
- DOI: 10.1159/000525300
Ferric Citrate Hydrate Has Little Impact on Hyperplasia of Enterochromaffin Cells in the Rat Small Intestine Compared to Sodium Ferrous Citrate
Abstract
Introduction: The most detrimental factor preventing the use of oral iron in the treatment of iron deficiency anemia is gastrointestinal side effects accompanied by nausea and vomiting. Anorexia is a known secondary effect of nausea and vomiting. The important gastrointestinal signaling molecule 5-hydroxytryptamine (5-HT) is critically involved in not only physiological function but also nausea and vomiting. The present study was designed to compare the effects of the administration of sodium ferrous citrate (SF) and ferric citrate hydrate (FC) to rats on anorexia and hyperplasia of enterochromaffin cells, which mainly synthesize and store 5-HT.
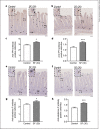
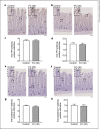
Methods: Rats received either SF (3 or 30 mg/kg/day) or FC (30 mg/kg/day) orally for 4 days. Food and water intakes were measured every 24 h during the study. At 96 h after the first administration of the oral iron preparation, the duodenal and jejunal tissues were collected for analysis. Enterochromaffin cells were detected by immunohistochemical analysis.
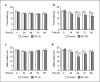
Results: Administration of 3 mg/kg SF had no effect on anorexia but led to increased hyperplasia of enterochromaffin cells in the duodenum (p < 0.1). Administration of 30 mg/kg SF significantly decreased food and water intakes and significantly increased hyperplasia of enterochromaffin cells in the duodenum and jejunum. Alternatively, administration of 30 mg/kg FC had no significant effect on food and water intakes or hyperplasia of enterochromaffin cells.
Conclusion: The lower impact on the hyperplasia of enterochromaffin cells of FC compared to SF may contribute to the maintenance of rats' physical condition.
Keywords: Anorexia; Enterochromaffin cells; Ferric citrate hydrate; Sodium ferrous citrate; Tryptophan hydroxylase.
The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Pasricha SR, Tye-Din J, Muckenthaler MU, Swinkels DW. Iron deficiency. Lancet. 2021;397((10270)):233–248. - PubMed
-
- Hoppe JO, Marcelli GM, Tainter ML. A review of the toxicity of iron compounds. Am J Med Sci. 1955;230:558–571. - PubMed
-
- Souza AI, Batista Filho MB, Bresani CC, Ferreira LOC, Figueiroa JN. Adherence and side effects of three ferrous sulfate treatment regimens on anemic pregnant women in clinical trials. Cad Saude Publica. 2009;25:1225–1233. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

