Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial
- PMID: 36007534
- PMCID: PMC9418415
- DOI: 10.1016/S0140-6736(22)01472-6
Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial
Erratum in
-
Department of Error.Lancet. 2022 Sep 10;400(10355):810. doi: 10.1016/S0140-6736(22)01661-0. Epub 2022 Aug 29. Lancet. 2022. PMID: 36049494 Free PMC article. No abstract available.
Abstract
Background: Diabetic peripheral neuropathic pain (DPNP) is common and often distressing. Most guidelines recommend amitriptyline, duloxetine, pregabalin, or gabapentin as initial analgesic treatment for DPNP, but there is little comparative evidence on which one is best or whether they should be combined. We aimed to assess the efficacy and tolerability of different combinations of first-line drugs for treatment of DPNP.
Methods: OPTION-DM was a multicentre, randomised, double-blind, crossover trial in patients with DPNP with mean daily pain numerical rating scale (NRS) of 4 or higher (scale is 0-10) from 13 UK centres. Participants were randomly assigned (1:1:1:1:1:1), with a predetermined randomisation schedule stratified by site using permuted blocks of size six or 12, to receive one of six ordered sequences of the three treatment pathways: amitriptyline supplemented with pregabalin (A-P), pregabalin supplemented with amitriptyline (P-A), and duloxetine supplemented with pregabalin (D-P), each pathway lasting 16 weeks. Monotherapy was given for 6 weeks and was supplemented with the combination medication if there was suboptimal pain relief (NRS >3), reflecting current clinical practice. Both treatments were titrated towards maximum tolerated dose (75 mg per day for amitriptyline, 120 mg per day for duloxetine, and 600 mg per day for pregabalin). The primary outcome was the difference in 7-day average daily pain during the final week of each pathway. This trial is registered with ISRCTN, ISRCTN17545443.
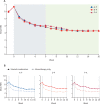
Findings: Between Nov 14, 2017, and July 29, 2019, 252 patients were screened, 140 patients were randomly assigned, and 130 started a treatment pathway (with 84 completing at least two pathways) and were analysed for the primary outcome. The 7-day average NRS scores at week 16 decreased from a mean 6·6 (SD 1·5) at baseline to 3·3 (1·8) at week 16 in all three pathways. The mean difference was -0·1 (98·3% CI -0·5 to 0·3) for D-P versus A-P, -0·1 (-0·5 to 0·3) for P-A versus A-P, and 0·0 (-0·4 to 0·4) for P-A versus D-P, and thus not significant. Mean NRS reduction in patients on combination therapy was greater than in those who remained on monotherapy (1·0 [SD 1·3] vs 0·2 [1·5]). Adverse events were predictable for the monotherapies: we observed a significant increase in dizziness in the P-A pathway, nausea in the D-P pathway, and dry mouth in the A-P pathway.
Interpretation: To our knowledge, this was the largest and longest ever, head-to-head, crossover neuropathic pain trial. We showed that all three treatment pathways and monotherapies had similar analgesic efficacy. Combination treatment was well tolerated and led to improved pain relief in patients with suboptimal pain control with a monotherapy.
Funding: National Institute for Health Research (NIHR) Health Technology Assessment programme.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests ST reports honoraria for educational meetings from Pfizer, Viatris, Wörwag Pharma, Novo Nordisk, Merck & Co, Eva Pharma, Hikma Pharmaceuticals, Abbott Laboratories, AstraZeneca, Nevro, Procter & Gamble Health, Astellas Pharma, and Berlin-Chemi; consulting fees from Bayer, NeuroPn Therapeutics, Wörwag Pharma, Angelini, Grünenthal, TRIGOcare International, Nevro Mitsubishi Tanabe Pharma Corporation, and Confo Therapeutics; and a research grant from Procter and Gamble Health paid to Sheffield Teaching Hospitals. UA reports honoraria for educational meetings from Eli Lilly, Napp Pharmaceuticals, Sanofi, and Boehringer Ingelheim. EBJ reports honoraria and research support from Novo Nordisk and Sanofi. SHA reports honoraria for educational meetings from Novo Nordisk, Eli Lilly, and Sanofi. PV reports honoraria from Merck and Sanofi. MJ reports honoraria for advisory boards and speaker fees from Grünenthal (2015 to present) and being a co-chairperson, since 2014, of the Chronic Pain Policy Coalition and a council (2012 to present) and ordinary member of the British Pain Society. DB reports grants and contracts from Novartis, Grunenthal, Bayer, and Air Liquide; and consulting fees from Bayer and Air Liquide. DLB has acted as a consultant on behalf of Oxford University Innovation for AditumBio, Amgen, Bristows, Latigo Biotherapeutics, GlaxoSmithKline, Ionis Pharmaceuticals, Eli Lilly, OliPass, Regeneron Pharmaceuticals, and Theranexus, over the past 2 years; has received research funding from Eli Lilly and AstraZeneca; has received an industrial partnership grant from the Biotechnology and Biological Sciences Research Council and AstraZeneca; and reports grants and contracts for several studies from the UK Research and Innovation, Medical Research Council (MRC), Action Medical Research for Children, MRC Research Grant, Wellcome Trust Senior Clinical Scientist Fellowship, Novo Nordisk Foundation, EU Horizon 2020, MRC Clinical Research Training Fellowship, and Wellcome Trust Strategic Award. DS reports membership of the advisory boards of Impeto Medical, PelliTec, and FeetMe. CC is a member of the NIHR Clinical Trial Unit (CTU) Support Funding Committee, NIHR CTU Standing Advisory Committee, NIHR Programme Grants for Applied Research Subcommittee, and Trial Steering Committees for other NIHR-funded trials. All other authors declare no competing interests.
Figures
Comment in
-
Effective treatment pathways exist for DPNP.Lancet. 2022 Aug 27;400(10353):639-641. doi: 10.1016/S0140-6736(22)01526-4. Epub 2022 Aug 22. Lancet. 2022. PMID: 36007533 No abstract available.
-
Who really benefits from drug combinations and long titrations for pain?Lancet. 2023 Jan 21;401(10372):192. doi: 10.1016/S0140-6736(23)00003-X. Lancet. 2023. PMID: 36681411 No abstract available.
-
Diabetische Neuropathie: Kombi-Therapien wirken besser.MMW Fortschr Med. 2023 Mar;165(4):26-27. doi: 10.1007/s15006-023-2392-3. MMW Fortschr Med. 2023. PMID: 36826651 German. No abstract available.
References
-
- Sloan G, Selvarajah D, Tesfaye S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat Rev Endocrinol. 2021;17:400–420. - PubMed
-
- Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352:341–350. - PubMed
-
- Sadosky A, Mardekian J, Parsons B, Hopps M, Bienen EJ, Markman J. Healthcare utilization and costs in diabetes relative to the clinical spectrum of painful diabetic peripheral neuropathy. J Diabetes Complications. 2015;29:212–217. - PubMed
-
- National Institute for Health and Care Excellence Neuropathic pain in adults: pharmacological management in non-specialist settings. (CG 173) 2021. https://www.nice.org.uk/guidance/cg173 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

