Effect of alcohol exposure on the efficacy and safety of tenofovir alafenamide fumarate, a major medicine against human immunodeficiency virus
- PMID: 36007574
- PMCID: PMC10225116
- DOI: 10.1016/j.bcp.2022.115224
Effect of alcohol exposure on the efficacy and safety of tenofovir alafenamide fumarate, a major medicine against human immunodeficiency virus
Abstract
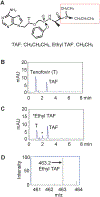
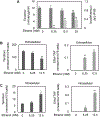
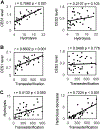
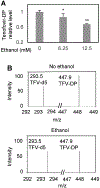
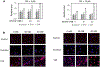
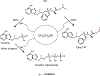
Human immunodeficiency virus (HIV) continues to be a major health concern. AIDS-related deaths (acquired immunodeficiency syndrome) have decreased recently, but chronic liver disease is now a major cause of mortality among HIV patients. Widespread alcohol use is recognized to be a major contributing factor. Tenofovir alafenamide fumarate (TAF), one of the most used HIV drugs, requires hydrolysis followed by phosphorylation to produce tenofovir diphosphate, the ultimate anti-HIV metabolite. Carboxylesterase-1 (CES1), established to hydrolyze TAF, is known to catalyze transesterification in the presence of ethanol. The aim of the study was to test the hypothesis that metabolism-based interactions between TAF and ethanol negatively impact both efficacy and safety of TAF. To test this hypothesis, the metabolism of TAF was determined in human primary hepatocytes and with a large number of human liver samples (S9 fractions) in the presence or absence of ethanol. The metabolism was monitored by LC-MS/MS (liquid chromatography with tandem mass spectrometry) and the level of CES1 or CES2 was determined by Western blotting. Consistent with the hypothesis, TAF underwent transesterification in the presence of ethanol accompanied by decreased hydrolysis. The formation of tenofovir diphosphate (the therapeutically active metabolite) was significantly decreased. In addition, TAF but not its hydrolytic metabolite, was found to increase intracellular lipid retention, and the increase was enhanced by ethanol. These findings conclude that alcohol consumption, beyond commonly accepted poor adherence to HIV medications, directly impacts the efficacy and safety of TAF.
Keywords: Acquired immunodeficiency syndrome; Carboxylesterase-1 (CES1); Human immunodeficiency virus; Liver toxicity; Tenofovir alafenamide fumarate.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Global Health Observatory (GHO) data (2021). Available from: <https://www.who.int/data/gho/data/themes/hiv-aids>.
-
- Global Aids update. Available from: <https://www.unaids.org/en/resources/documents/2021/2021-global-aids-update>.
-
- HIV in the United States and Dependent Areas. (2019). https://www.cdc.gov ⟩ hiv ⟩ statistics ⟩ overview ⟩ ataglance.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

