The acyclotide ribe 31 from Rinorea bengalensis has selective cytotoxicity and potent insecticidal properties in Drosophila
- PMID: 36007611
- PMCID: PMC9513267
- DOI: 10.1016/j.jbc.2022.102413
The acyclotide ribe 31 from Rinorea bengalensis has selective cytotoxicity and potent insecticidal properties in Drosophila
Abstract
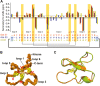
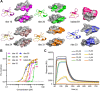
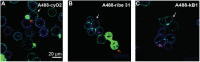
Cyclotides and acyclic versions of cyclotides (acyclotides) are peptides involved in plant defense. These peptides contain a cystine knot motif formed by three interlocked disulfide bonds, with the main difference between the two classes being the presence or absence of a cyclic backbone, respectively. The insecticidal activity of cyclotides is well documented, but no study to date explores the insecticidal activity of acyclotides. Here, we present the first in vivo evaluation of the insecticidal activity of acyclotides from Rinorea bengalensis on the vinegar fly Drosophila melanogaster. Of a group of structurally comparable acyclotides, ribe 31 showed the most potent toxicity when fed to D. melanogaster. We screened a range of acyclotides and cyclotides and found their toxicity toward human red blood cells was substantially lower than toward insect cells, highlighting their selectivity and potential for use as bioinsecticides. Our confocal microscopy experiments indicated their cytotoxicity is likely mediated via membrane disruption. Furthermore, our surface plasmon resonance studies suggested ribe 31 preferentially binds to membranes containing phospholipids with phosphatidyl-ethanolamine headgroups. Despite having an acyclic backbone, we determined the three-dimensional NMR solution structure of ribe 31 is similar to that of cyclotides. In summary, our results suggest that, with further optimization, ribe 31 could have applications as an insecticide due to its potent in vivo activity against D. melanogaster. More broadly, this work advances the field by demonstrating that acyclotides are more common than previously thought, have potent insecticidal activity, and have the advantage of potentially being more easily manufactured than cyclotides.
Keywords: Drosophila melanogaster; Rinorea bengalensis; acyclotides; bioinsecticides; cyclotides; cytotoxic.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Craik D.J., Daly N.L., Bond T., Waine C. Plant cyclotides: a unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J. Mol. Biol. 1999;294:1327–1336. - PubMed
-
- de Veer S.J., Kan M.W., Craik D.J. Cyclotides: from structure to function. Chem. Rev. 2019;119:12375–12421. - PubMed
-
- Wang C.K., Craik D.J. Designing macrocyclic disulfide-rich peptides for biotechnological applications. Nat. Chem. Biol. 2018;14:417–427. - PubMed
-
- Colgrave M.L., Craik D.J. Thermal, chemical, and enzymatic stability of the cyclotide kalata B1: the importance of the cyclic cystine knot. Biochemistry. 2004;43:5965–5975. - PubMed
-
- Hernandez J.F., Gagnon J., Chiche L., Nguyen T.M., Andrieu J.P., Heitz A., et al. Squash trypsin inhibitors from Momordica cochinchinensis exhibit an atypical macrocyclic structure. Biochemistry. 2000;39:5722–5730. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

