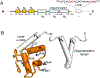Terminase Subunits from the Pseudomonas-Phage E217
- PMID: 36007626
- PMCID: PMC10026623
- DOI: 10.1016/j.jmb.2022.167799
Terminase Subunits from the Pseudomonas-Phage E217
Abstract
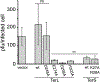
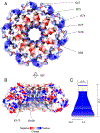
Pseudomonas phages are increasingly important biomedicines for phage therapy, but little is known about how these viruses package DNA. This paper explores the terminase subunits from the Myoviridae E217, a Pseudomonas-phage used in an experimental cocktail to eradicate P. aeruginosa in vitro and in animal models. We identified the large (TerL) and small (TerS) terminase subunits in two genes ∼58 kbs away from each other in the E217 genome. TerL presents a classical two-domain architecture, consisting of an N-terminal ATPase and C-terminal nuclease domain arranged into a bean-shaped tertiary structure. A 2.05 Å crystal structure of the C-terminal domain revealed an RNase H-like fold with two magnesium ions in the nuclease active site. Mutations in TerL residues involved in magnesium coordination had a dominant-negative effect on phage growth. However, the two ions identified in the active site were too far from each other to promote two-metal-ion catalysis, suggesting a conformational change is required for nuclease activity. We also determined a 3.38 Å cryo-EM reconstruction of E217 TerS that revealed a ring-like decamer, departing from the most common nonameric quaternary structure observed thus far. E217 TerS contains both N-terminal helix-turn-helix motifs enriched in basic residues and a central channel lined with basic residues large enough to accommodate double-stranded DNA. Overexpression of TerS caused a more than a 4-fold reduction of E217 burst size, suggesting a catalytic amount of the protein is required for packaging. Together, these data expand the molecular repertoire of viral terminase subunits to Pseudomonas-phages used for phage therapy.
Keywords: Pseudomonas-phages; bacteriophage E217; large terminase; small terminase; viral genome-packaging motor.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures