Physical-Chemical Regulation of Membrane Receptors Dynamics in Viral Invasion and Immune Defense
- PMID: 36007627
- PMCID: PMC9394170
- DOI: 10.1016/j.jmb.2022.167800
Physical-Chemical Regulation of Membrane Receptors Dynamics in Viral Invasion and Immune Defense
Abstract
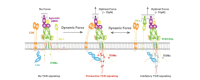
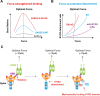
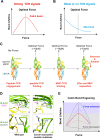
Mechanical cues dynamically regulate membrane receptors functions to trigger various physiological and pathological processes from viral invasion to immune defense. These cues mainly include various types of dynamic mechanical forces and the spatial confinement of plasma membrane. However, the molecular mechanisms of how they couple with biochemical cues in regulating membrane receptors functions still remain mysterious. Here, we review recent advances in methodologies of single-molecule biomechanical techniques and in novel biomechanical regulatory mechanisms of critical ligand recognition of viral and immune receptors including SARS-CoV-2 spike protein, T cell receptor (TCR) and other co-stimulatory immune receptors. Furthermore, we provide our perspectives of the general principle of how force-dependent kinetics determine the dynamic functions of membrane receptors and of biomechanical-mechanism-driven SARS-CoV-2 neutralizing antibody design and TCR engineering for T-cell-based therapies.
Keywords: immune defense; mechanical force; membrane receptors dynamics; spatial confinement of plasma membrane; viral invasion.
Copyright © 2022. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Interest Authors declare that they have no competing interests.
Figures

References
-
- Mørch A.M., Bálint Š., Santos A.M., Davis S.J., Dustin M.L. Coreceptors and TCR Signaling – the Strong and the Weak of It. Front Cell Dev Biol. 2020:1–13. https://www.frontiersin.org/ - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

