A mechanistic analysis of bacterial recognition and serine protease cascade initiation in larval hemolymph of Manduca sexta
- PMID: 36007679
- PMCID: PMC9890636
- DOI: 10.1016/j.ibmb.2022.103818
A mechanistic analysis of bacterial recognition and serine protease cascade initiation in larval hemolymph of Manduca sexta
Abstract
Serine protease cascades have evolved in vertebrates and invertebrates to mediate rapid defense responses. Previous biochemical studies showed that in hemolymph of a caterpillar, Manduca sexta, recognition of fungi by β-1,3-glucan recognition proteins (βGRP1 and βGRP2) or recognition of bacteria by peptidoglycan recognition protein-1 (PGRP1) and microbe binding protein (MBP) results in autoactivation of hemolymph protease-14 precursor (proHP14). HP14 then activates downstream members of a protease cascade leading to the melanization immune response. ProHP14 has a complex domain architecture, with five low-density lipoprotein receptor class A repeats at its amino terminus, followed by a Sushi domain, a Sushi domain variant called Wonton, and a carboxyl-terminal serine protease catalytic domain. Its zymogen form is activated by specific proteolytic cleavage at the amino-terminal end of the protease domain. While a molecular mechanism for recognition and triggering the response to β-1,3-glucan has been delineated, it is unclear how bacterial recognition stimulates proHP14 activation. To fill this knowledge gap, we expressed the two domains of M. sexta MBP and found that the amino-terminal domain binds to diaminopimelic acid-peptidoglycan (DAP-PG). ProHP14 bound to both the carboxyl-terminal domain (MBP-C) and amino-terminal domain (MBP-N) of MBP. In the mixture of DAP-PG, MBP, and larval plasma, inclusion of an HP14 fragment composed of LDLa repeats 2-5 (LDLa2-5) or MBP-C significantly reduced prophenoloxidase activation, likely by competing with the interactions of the full-length proteins, and suggesting that molecular interactions involving these regions of proHP14 and MBP take part in proHP14 activation in response to peptidoglycan. Using a series of N-terminally truncated versions of proHP14, we found that autoactivation required LDLa2-5. The optimal ratio of PGRP1, MBP, and proHP14 is close to 3:2:1. In summary, proHP14 autoactivation by DAP-type peptidoglycan requires binding of DAP-PG by PGRP1 and the MBP N-terminal domain and association of the LDLa2-5 region of proHP14 with the MBP C-terminal domain. These interactions may concentrate the proHP14 zymogen at the bacterial cell wall surface and promote autoactivation.
Keywords: Hemolymph proteins; Insect immunity; Melanization; Microbe binding protein; Pattern recognition; Peptidoglycan; Peptidoglycan recognition protein; Toll pathway.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Figures





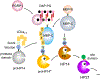
References
-
- Choteau L, Parny M, François N, Bertin B, Fumery M, Dubuquoy L, Takahashi K, Colombel J-F, Jouault T, Poulain D, Sendid B, Jawhara S, 2016. Role of mannose-binding lectin in intestinal homeostasis and fungal elimination. Mucosal Immunol. 9, 767–776. - PubMed
-
- Dai H, Hiromasa Y, Takahashi D, VanderVelde D, Fabrick JA, Kanost MR, Krishnamoorthi R, 2013. An initial event in the insect innate immune response: structural and biological studies of interactions between β-1,3-glucan and the N-terminal domain of β-1,3-glucan recognition protein. Biochemistry 52, 161–170. - PMC - PubMed
-
- Dunn P, Drake D, 1983. Fate of bacteria injected into naïve and immunized larvae of the tobacco hornworm, Manduca sexta. J. Invert. Pathol 41, 77–85.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

