Substrate-based approaches in ventricular tachycardia ablation
- PMID: 36007824
- PMCID: PMC9649336
- DOI: 10.1016/j.ipej.2022.08.002
Substrate-based approaches in ventricular tachycardia ablation
Abstract
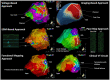
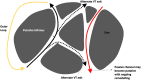
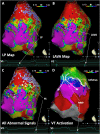
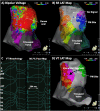
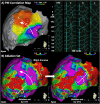
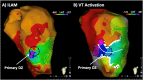
Catheter ablation for ventricular tachycardia (VT) in patients with structural heart disease is now part of standard care. Mapping and ablation of the clinical VT is often limited when the VT is noninducible, nonsustained or not haemodynamically tolerated. Substrate-based ablation strategies have been developed in an aim to treat VT in this setting and, subsequently, have been shown to improve outcomes in VT ablation when compared to focused ablation of mapped VTs. Since the initial description of linear ablation lines targeting ventricular scar, many different approaches to substrate-based VT ablation have been developed. Strategies can broadly be divided into three categories: 1) targeting abnormal electrograms, 2) anatomical targeting of conduction channels between areas of myocardial scar, and 3) targeting areas of slow and/or decremental conduction, identified with "functional" substrate mapping techniques. This review summarises contemporary substrate-based ablation strategies, along with their strengths and weaknesses.
Keywords: Functional substrate mapping; ILAM; Ventricular arrhythmias; Ventricular tachycardia.
Copyright © 2022 Indian Heart Rhythm Society. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest Saurabh Kumar has received honoraria from Biosense Webster, Abbott Medical, Biotronik, and Sanofi Aventis. Jonathan Kalman is supported by a National Health and Medical Research Council of Australia practitioner fellowship, and has received research and fellowship support from Biosense Webster, Abbott and Medtronic. Geoffrey Lee has received consulting fees and speaker honoraria from Biosense Webster. Other authors have no discloses.
Figures

Similar articles
-
The value of functional substrate mapping in ventricular tachycardia ablation.Heart Rhythm O2. 2022 Nov 2;4(2):134-146. doi: 10.1016/j.hroo.2022.10.013. eCollection 2023 Feb. Heart Rhythm O2. 2022. PMID: 36873315 Free PMC article. Review.
-
Substrate mapping for unstable ventricular tachycardia.Heart Rhythm. 2016 Feb;13(2):569-83. doi: 10.1016/j.hrthm.2015.09.023. Epub 2015 Sep 26. Heart Rhythm. 2016. PMID: 26410105 Review.
-
Rotational Activation Pattern During Functional Substrate Mapping: Novel Target for Catheter Ablation of Scar-Related Ventricular Tachycardia.Circ Arrhythm Electrophysiol. 2022 Jan;15(1):e010308. doi: 10.1161/CIRCEP.121.010308. Epub 2021 Dec 23. Circ Arrhythm Electrophysiol. 2022. PMID: 34937390 Free PMC article.
-
Substrate characterization and catheter ablation in patients with scar-related ventricular tachycardia using ultra high-density 3-D mapping.J Cardiovasc Electrophysiol. 2017 Sep;28(9):1058-1067. doi: 10.1111/jce.13270. Epub 2017 Jul 3. J Cardiovasc Electrophysiol. 2017. PMID: 28597532
-
Noninvasive epicardial and endocardial electrocardiographic imaging of scar-related ventricular tachycardia.J Electrocardiol. 2016 Nov-Dec;49(6):887-893. doi: 10.1016/j.jelectrocard.2016.07.026. Epub 2016 Jul 28. J Electrocardiol. 2016. PMID: 27968777 Free PMC article.
Cited by
-
The integration of electroanatomic maps into cardiac radioablation treatment planning: A systematic review.Med Phys. 2025 Aug;52(8):e18010. doi: 10.1002/mp.18010. Med Phys. 2025. PMID: 40781792 Free PMC article. Review.
-
Entrapment of high-density grid mapping catheter in a percutaneous ventricular assist device pigtail during ventricular tachycardia ablation with percutaneous hemodynamic support.HeartRhythm Case Rep. 2023 May 2;9(7):478-481. doi: 10.1016/j.hrcr.2023.04.014. eCollection 2023 Jul. HeartRhythm Case Rep. 2023. PMID: 37492057 Free PMC article. No abstract available.
-
Integration of automated peak frequency annotation with voltage mapping for identifying ventricular tachycardia ablation sites.J Interv Card Electrophysiol. 2025 Apr 16. doi: 10.1007/s10840-025-02045-4. Online ahead of print. J Interv Card Electrophysiol. 2025. PMID: 40237974
-
An annotation-independent algorithm based on electrogram characteristics to guide the identification of ventricular tachycardia isthmuses in patients with structural heart disease.J Interv Card Electrophysiol. 2024 Jun;67(4):739-750. doi: 10.1007/s10840-023-01657-y. Epub 2023 Sep 30. J Interv Card Electrophysiol. 2024. PMID: 37775727
-
How Imaging Techniques Improve Ventricular Arrhythmia Ablation: A Multimodality-Based Approach.J Clin Med. 2023 Nov 30;12(23):7420. doi: 10.3390/jcm12237420. J Clin Med. 2023. PMID: 38068472 Free PMC article. Review.
References
-
- Arenal Pá Ángel, Jiménez-Candil Javier, Tercedor Luis, Calvo David, Arribas Fernando, Fernández-Portales Javier, Merino José Luis, Hernández-Madrid Antonio, Fernández-Avilés Francisco J., Berruezo Antonio. Substrate ablation vs antiarrhythmic drug therapy for symptomatic ventricular tachycardia. J Am Coll Cardiol. 2022;79:1441–1453. - PubMed
-
- Sapp J.L., Wells G.A., Parkash R., Stevenson W.G., Blier L., Sarrazin J.F., et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med. 2016;375(2):111–121. - PubMed
-
- Tung R. Challenges and pitfalls of entrainment mapping of ventricular tachycardia: ten illustrative concepts. Circ Arrhythm Electrophysiol. 2017;10(4) - PubMed
-
- Josephson M.E., Horowitz L.N., Farshidi A., Spielman S.R., Michelson E.L., Greenspan A.M. Sustained ventricular tachycardia: evidence for protected localized reentry. Am J Cardiol. 1978;42(3):416–424. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
