Outpatient convalescent plasma therapy for high-risk patients with early COVID-19: a randomized placebo-controlled trial
- PMID: 36007870
- PMCID: PMC9395229
- DOI: 10.1016/j.cmi.2022.08.005
Outpatient convalescent plasma therapy for high-risk patients with early COVID-19: a randomized placebo-controlled trial
Abstract
Objectives: The potential benefit of convalescent plasma (CP) therapy for coronavirus disease 2019 (COVID-19) is highest when administered early after symptom onset. Our objective was to determine the effectiveness of CP therapy in improving the disease course of COVID-19 among high-risk outpatients.
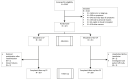
Methods: A multicentre, double-blind randomized trial was conducted comparing 300 mL of CP with non-CP. Patients were ≥50 years, were symptomatic for <8 days, had confirmed RT-PCR or antigen test result for COVID-19 and had at least one risk factor for severe COVID-19. The primary endpoint was the highest score on a 5-point ordinal scale ranging from fully recovered (score = 1) or not (score = 2) on day 7, over hospital admission (score = 3), intensive care unit admission (score = 4) and death (score = 5) in the 28 days following randomization. Secondary endpoints were hospital admission, symptom duration and viral RNA excretion.
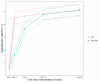
Results: After the enrolment of 421 patients and the transfusion in 416 patients, recruitment was discontinued when the countrywide vaccination uptake in those aged >50 years was 80%. Patients had a median age of 60 years, symptoms for 5 days, and 207 of 416 patients received CP therapy. During the 28 day follow-up, 28 patients were hospitalized and two died. The OR for an improved disease severity score with CP was 0.86 (95% credible interval, 0.59-1.22). The OR was 0.58 (95% CI, 0.33-1.02) for patients with ≤5 days of symptoms. The hazard ratio for hospital admission was 0.61 (95% CI, 0.28-1.34). No difference was found in viral RNA excretion or in the duration of symptoms.
Conclusions: In patients with early COVID-19, CP therapy did not improve the 5-point disease severity score.
Keywords: Antibodies; COVID-19; Convalescent plasma; Outpatients; Therapy.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
COVID-19 convalescent plasma therapy through the lens of the third year of the pandemic.Clin Microbiol Infect. 2023 Feb;29(2):130-132. doi: 10.1016/j.cmi.2022.10.033. Epub 2022 Nov 4. Clin Microbiol Infect. 2023. PMID: 36343900 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

