Role of coagulation in persistent renal ischemia following reperfusion in an animal model
- PMID: 36007891
- PMCID: PMC9602917
- DOI: 10.1152/ajprenal.00162.2022
Role of coagulation in persistent renal ischemia following reperfusion in an animal model
Abstract
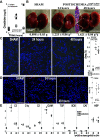
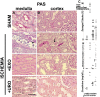
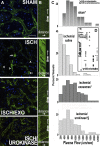
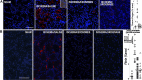
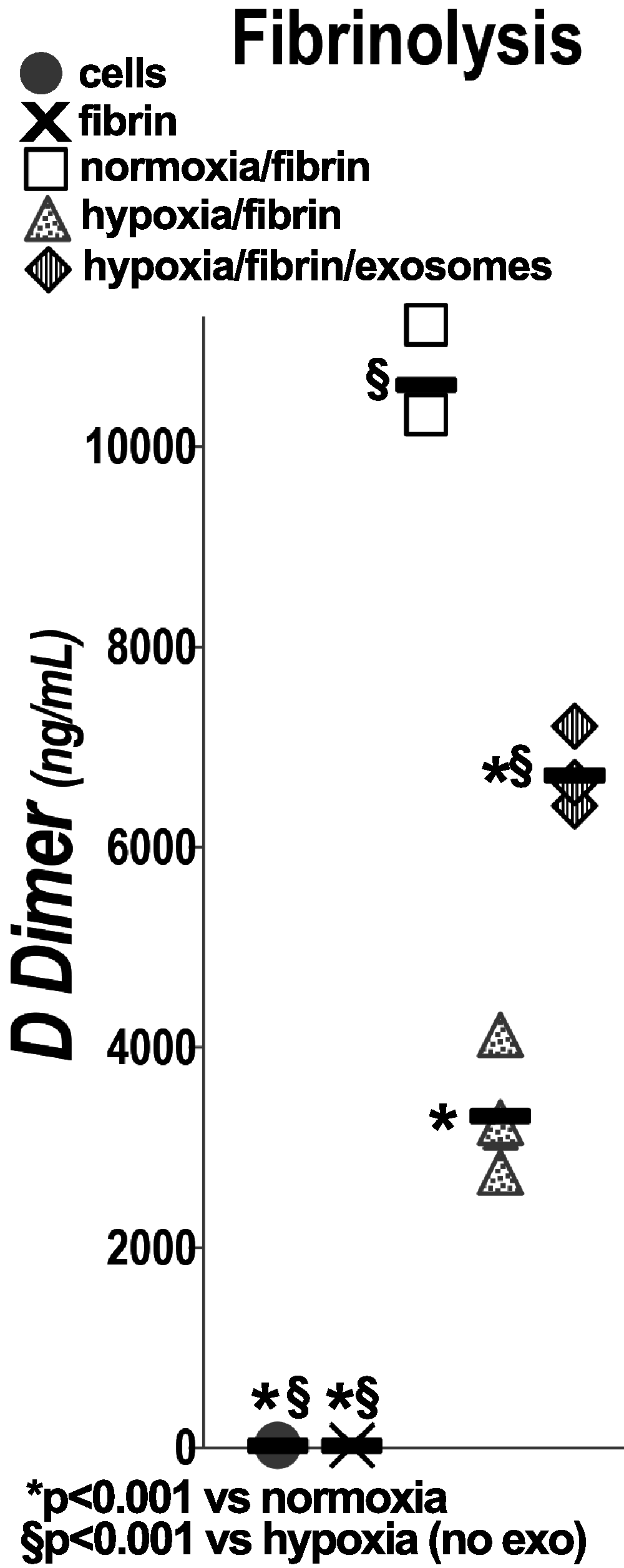
Ischemic acute kidney injury is common, deadly, and accelerates the progression of chronic kidney disease, yet has no specific therapy. After ischemia, reperfusion is patchy with early and persistent impairment in regional renal blood flow and cellular injury. We tested the hypothesis that intrarenal coagulation results in sustained renal ischemia following reperfusion, using a well-characterized model. Markedly decreased, but heterogeneous, microvascular plasma flow with microthrombi was found postischemia by intravital microscopy. Widespread tissue factor expression and fibrin deposition were also apparent. Clotting was accompanied by complement activation and inflammation. Treatment with exosomes derived from renal tubular cells or with the fibrinolytic urokinase, given 24 h postischemia when renal failure was established, significantly improved microvascular flow, coagulation, serum creatinine, and histological evidence of injury. These data support the hypothesis that intrarenal clotting occurs early and the resultant sustained ischemia is a critical determinant of renal failure following ischemia; they demonstrate that the coagulation abnormalities are amenable to therapy and that therapy results in improvement in both function and postischemic inflammation.NEW & NOTEWORTHY Ischemic renal injury carries very high morbidity and mortality, yet has no specific therapy. We found markedly decreased, heterogeneous microvascular plasma flow, tissue factor induction, fibrin deposition, and microthrombi after renal ischemia-reperfusion using a well-characterized model. Renal exosomes or the fibrinolytic urokinase, administered after renal failure was established, improved microvascular flow, coagulation, renal function, and histology. Data demonstrate that intrarenal clotting results in sustained ischemia amenable to therapy that improves both function and postischemic inflammation.
Keywords: acute kidney injury; exosomes; fibrinolysis; ischemia-reperfusion; microvasculature.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



Similar articles
-
Role of the plasma cascade systems in ischemia/reperfusion injury of bone.Bone. 2017 Apr;97:278-286. doi: 10.1016/j.bone.2016.12.007. Epub 2017 Jan 31. Bone. 2017. PMID: 28159709
-
Enhancement of endogenous fibrinolysis does not reduce local fibrin deposition, but modulates inflammation upon intestinal ischemia and reperfusion.Thromb Haemost. 2004 Mar;91(3):497-505. doi: 10.1160/TH03-08-0529. Thromb Haemost. 2004. PMID: 14983225
-
Caspase-3-dependent peritubular capillary dysfunction is pivotal for the transition from acute to chronic kidney disease after acute ischemia-reperfusion injury.Am J Physiol Renal Physiol. 2021 Sep 1;321(3):F335-F351. doi: 10.1152/ajprenal.00690.2020. Epub 2021 Aug 2. Am J Physiol Renal Physiol. 2021. PMID: 34338031
-
Coagulation, fibrinolysis, and fibrin deposition in acute lung injury.Crit Care Med. 2003 Apr;31(4 Suppl):S213-20. doi: 10.1097/01.CCM.0000057846.21303.AB. Crit Care Med. 2003. PMID: 12682443 Review.
-
[The role of macrophage polarization and interaction with renal tubular epithelial cells in ischemia-reperfusion induced acute kidney injury].Sheng Li Xue Bao. 2022 Feb 25;74(1):28-38. Sheng Li Xue Bao. 2022. PMID: 35199123 Review. Chinese.
Cited by
-
Renal, but not platelet or skin, extracellular vesicles decrease oxidative stress, enhance nascent peptide synthesis, and protect from ischemic renal injury.Am J Physiol Renal Physiol. 2023 Aug 1;325(2):F164-F176. doi: 10.1152/ajprenal.00321.2022. Epub 2023 Jun 15. Am J Physiol Renal Physiol. 2023. PMID: 37318988 Free PMC article.
-
Impaired microvascular circulation in distant organs following renal ischemia.PLoS One. 2023 Jun 2;18(6):e0286543. doi: 10.1371/journal.pone.0286543. eCollection 2023. PLoS One. 2023. PMID: 37267281 Free PMC article.
-
The Many Faces of Protease-Activated Receptor 2 in Kidney Injury.Biomedicines. 2025 Feb 8;13(2):414. doi: 10.3390/biomedicines13020414. Biomedicines. 2025. PMID: 40002827 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

