Magel2 knockdown in hypothalamic POMC neurons innervating the medial amygdala reduces susceptibility to diet-induced obesity
- PMID: 36007929
- PMCID: PMC9418835
- DOI: 10.26508/lsa.202201502
Magel2 knockdown in hypothalamic POMC neurons innervating the medial amygdala reduces susceptibility to diet-induced obesity
Abstract
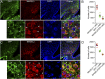
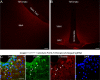
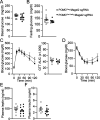
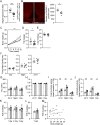
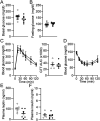
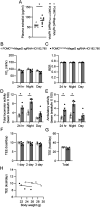
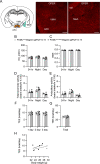
Hyperphagia and obesity profoundly affect the health of children with Prader-Willi syndrome (PWS). The Magel2 gene among the genes in the Prader-Willi syndrome deletion region is expressed in proopiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus (ARC). Knockout of the Magel2 gene disrupts POMC neuronal circuits and functions. Here, we report that loss of the Magel2 gene exclusively in ARCPOMC neurons innervating the medial amygdala (MeA) causes a reduction in body weight in both male and female mice fed with a high-fat diet. This anti-obesity effect is associated with an increased locomotor activity. There are no significant differences in glucose and insulin tolerance in mice without the Magel2 gene in ARCPOMC neurons innervating the MeA. Plasma estrogen levels are higher in female mutant mice than in controls. Blockade of the G protein-coupled estrogen receptor (GPER), but not estrogen receptor-α (ER-α), reduces locomotor activity in female mutant mice. Hence, our study provides evidence that knockdown of the Magel2 gene in ARCPOMC neurons innervating the MeA reduces susceptibility to diet-induced obesity with increased locomotor activity through activation of central GPER.
© 2022 Choi et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Progressive postnatal decline in leptin sensitivity of arcuate hypothalamic neurons in the Magel2-null mouse model of Prader-Willi syndrome.Hum Mol Genet. 2015 Aug 1;24(15):4276-83. doi: 10.1093/hmg/ddv159. Epub 2015 Apr 29. Hum Mol Genet. 2015. PMID: 25926624
-
Magel2 is required for leptin-mediated depolarization of POMC neurons in the hypothalamic arcuate nucleus in mice.PLoS Genet. 2013;9(1):e1003207. doi: 10.1371/journal.pgen.1003207. Epub 2013 Jan 17. PLoS Genet. 2013. PMID: 23341784 Free PMC article.
-
Impaired melanocortin pathway function in Prader-Willi syndrome gene-Magel2 deficient mice.Hum Mol Genet. 2018 Sep 15;27(18):3129-3136. doi: 10.1093/hmg/ddy216. Hum Mol Genet. 2018. PMID: 29878108
-
Magel2 gene in hypothalamic POMC neurons influences the impact of stress on anxiety-like behavior and spatial learning associated with a food reward in male mice.bioRxiv [Preprint]. 2025 Aug 7:2025.07.09.663942. doi: 10.1101/2025.07.09.663942. bioRxiv. 2025. PMID: 40766483 Free PMC article. Preprint.
-
MAGEL2-related disorders: A study and case series.Clin Genet. 2019 Dec;96(6):493-505. doi: 10.1111/cge.13620. Epub 2019 Aug 22. Clin Genet. 2019. PMID: 31397880 Free PMC article.
Cited by
-
Illuminating the druggable genome: Pathways to progress.Drug Discov Today. 2024 Mar;29(3):103805. doi: 10.1016/j.drudis.2023.103805. Epub 2023 Oct 27. Drug Discov Today. 2024. PMID: 37890715 Free PMC article. Review.
-
Sex dimorphic associations of Prader-Willi imprinted gene expressions in umbilical cord with prenatal and postnatal growth in healthy infants.World J Pediatr. 2025 Jan;21(1):100-112. doi: 10.1007/s12519-024-00865-4. Epub 2025 Jan 22. World J Pediatr. 2025. PMID: 39838229 Free PMC article.
-
The Role of the Arcuate Nucleus in Regulating Hunger and Satiety in Prader-Willi Syndrome.Curr Issues Mol Biol. 2025 Mar 14;47(3):192. doi: 10.3390/cimb47030192. Curr Issues Mol Biol. 2025. PMID: 40136445 Free PMC article. Review.
-
The expanding landscape of genetic causes of obesity.Pediatr Res. 2025 Mar;97(4):1358-1369. doi: 10.1038/s41390-024-03780-6. Epub 2024 Dec 17. Pediatr Res. 2025. PMID: 39690244 Review.
References
-
- Alfaro DL, Lemoine P, Ehlinger V, Molinas C, Diene G, Valette M, Pinto G, Coupaye M, Poitou-Bernert C, Thuilleaux D, et al. (2019) Causes of death in prader-willi syndrome: Lessons from 11 years’ experience of a national reference center. Orphanet J Rare Dis 14: 238. 10.1186/s13023-019-1214-2 - DOI - PMC - PubMed
-
- Biglari N, Gaziano I, Schumacher J, Radermacher J, Paeger L, Klemm P, Chen W, Corneliussen S, Wunderlich CM, Sue M, et al. (2021) Functionally distinct pomc-expressing neuron subpopulations in hypothalamus revealed by intersectional targeting. Nat Neurosci 24: 913–929. 10.1038/s41593-021-00854-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
