Multimodality Imaging in Primary Progressive Aphasia
- PMID: 36007947
- PMCID: PMC9451618
- DOI: 10.3174/ajnr.A7613
Multimodality Imaging in Primary Progressive Aphasia
Abstract
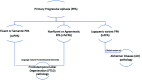
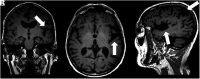
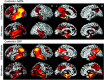
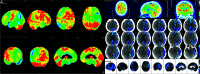
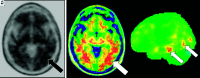
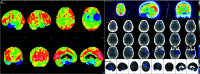
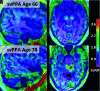
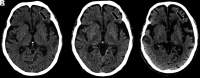
Primary progressive aphasia is a clinically and neuropathologically heterogeneous group of progressive neurodegenerative disorders, characterized by language-predominant impairment and commonly associated with atrophy of the dominant language hemisphere. While this clinical entity has been recognized dating back to the 19th century, important advances have been made in defining our current understanding of primary progressive aphasia, with 3 recognized subtypes to date: logopenic variant, semantic variant, and nonfluent/agrammatic variant. Given the ongoing progress in our understanding of the neurobiology and genomics of these rare neurodegenerative conditions, accurate imaging diagnoses are of the utmost importance and carry implications for future therapeutic triaging. This review covers the diverse spectrum of primary progressive aphasia and its multimodal imaging features, including structural, functional, and molecular neuroimaging findings; it also highlights currently recognized diagnostic criteria, clinical presentations, histopathologic biomarkers, and treatment options of these 3 primary progressive aphasia subtypes.
© 2022 by American Journal of Neuroradiology.
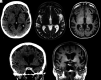
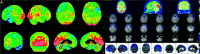
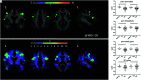
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
