Generation of evidence supporting the content validity of SF-36, FACIT-F, and LupusQoL, and novel patient-reported symptom items for use in patients with systemic lupus erythematosus (SLE) and SLE with lupus nephritis (LN)
- PMID: 36007978
- PMCID: PMC9422858
- DOI: 10.1136/lupus-2022-000712
Generation of evidence supporting the content validity of SF-36, FACIT-F, and LupusQoL, and novel patient-reported symptom items for use in patients with systemic lupus erythematosus (SLE) and SLE with lupus nephritis (LN)
Abstract
Objective: SLE and lupus nephritis (LN) have significant impacts on the health-related quality of life of patients living with the condition, which are important to capture from the patient's perspective using patient-reported outcomes (PROs). The objectives of this study were to evaluate the content validity of PROs commonly used in SLE and LN (36-Item Short Form Health Survey (SF-36), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) and Lupus Quality of Life (LupusQoL), as well as novel PRO symptom severity items measuring skin rash, joint pain, joint stiffness and swelling of the legs and/or feet, in both populations.
Methods: Qualitative, semi-structured, cognitive interviews were conducted with 48 participants (SLE=28, LN=20). Understanding and relevance of symptom and impact PRO concepts from existing PROs were assessed, alongside novel PRO symptom severity items with different recall periods (24 hours vs 7 days) and response scales (Numerical Rating Scale (NRS) vs Verbal Rating Scale). Interviews were conducted in multiple rounds to allow for modifications to the novel PRO items. Analysis of verbatim interview transcripts was performed.
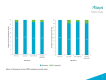
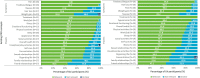
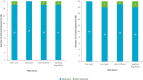
Results: Symptom and impact concepts assessed by the SF-36, FACIT-F, and LupusQoL were well understood by both participants with SLE and LN (≥90.0%), with most considered relevant by over half of the participants asked (≥51.9%). All participants asked (100%) understood the novel PRO symptom severity items, and the majority (≥90.0%) considered the symptoms relevant. Minor modifications to the novel PRO items were made between rounds to improve clarity based on participant feedback. The selected 7-day recall period and NRS in the final iteration of the PRO items were understood and relevant. No differences in interview findings between the SLE and LN samples were identified.
Conclusions: Findings provide evidence of content validity for concepts assessed by the SF-36, FACIT-F, LupusQoL and the novel PRO symptom severity items, supporting use of these PROs to comprehensively assess disease impact in future SLE and LN clinical trials.
Keywords: Lupus Nephritis; Outcome Assessment, Health Care; Qualitative Research; Quality of Life; Systemic Lupus Erythematosus.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RW-H, NW, MB, AR, AG, CT, HB and AW are employed by Adelphi Values, a health outcomes research company paid for conducting the research reported in this article. PB and PDL work for and own stocks/shares in Janssen Global Services LLC, which funded the research reported. EH and QZ work for and own stocks in Janssen R&D. ZT received funding from Schroeder Arthritis Institute and Lupus Ontario. VS received funding from numerous clients, including Janssen, which funded this research.
Figures
Similar articles
-
Patient-reported outcome measures for lupus nephritis: content validity of LupusQoL and FACIT-Fatigue.J Patient Rep Outcomes. 2024 Sep 30;8(1):115. doi: 10.1186/s41687-024-00783-z. J Patient Rep Outcomes. 2024. PMID: 39347839 Free PMC article.
-
Patient-reported outcome measures for systemic lupus erythematosus clinical trials: a review of content validity, face validity and psychometric performance.Health Qual Life Outcomes. 2014 Jul 22;12:116. doi: 10.1186/s12955-014-0116-1. Health Qual Life Outcomes. 2014. PMID: 25048687 Free PMC article. Review.
-
Measurement properties of selected patient-reported outcome measures for use in randomised controlled trials in patients with systemic lupus erythematosus: a systematic review.Lupus Sci Med. 2020 Jun;7(1):e000373. doi: 10.1136/lupus-2019-000373. Lupus Sci Med. 2020. PMID: 32591423 Free PMC article.
-
Psychometric Analysis from EMBODY1 and 2 Clinical Trials to Help Select Suitable Fatigue PRO Scales for Future Systemic Lupus Erythematosus Studies.Rheumatol Ther. 2021 Sep;8(3):1287-1301. doi: 10.1007/s40744-021-00338-4. Epub 2021 Jul 9. Rheumatol Ther. 2021. PMID: 34244970 Free PMC article.
-
Health-Related Quality of Life Improvements in Systemic Lupus Erythematosus Derived from a Digital Therapeutic Plus Tele-Health Coaching Intervention: Randomized Controlled Pilot Trial.J Med Internet Res. 2020 Oct 20;22(10):e23868. doi: 10.2196/23868. J Med Internet Res. 2020. PMID: 33079070 Free PMC article. Clinical Trial.
Cited by
-
Content validation of the SF-36v2® Health Survey Acute for use in hypoparathyroidism.Qual Life Res. 2023 Jun;32(6):1795-1806. doi: 10.1007/s11136-023-03352-x. Epub 2023 Feb 9. Qual Life Res. 2023. PMID: 36759379 Free PMC article.
-
German Translation and Linguistic Validation of the LIMB‑Q: A Patient-reported Outcome Measure for Lower Extremity Trauma.Plast Reconstr Surg Glob Open. 2024 Jul 19;12(7):e6001. doi: 10.1097/GOX.0000000000006001. eCollection 2024 Jul. Plast Reconstr Surg Glob Open. 2024. PMID: 39036594 Free PMC article.
-
Patient-reported outcome measures for lupus nephritis: content validity of LupusQoL and FACIT-Fatigue.J Patient Rep Outcomes. 2024 Sep 30;8(1):115. doi: 10.1186/s41687-024-00783-z. J Patient Rep Outcomes. 2024. PMID: 39347839 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
