Asthma control with ICS-formoterol reliever versus maintenance ICS and SABA reliever therapy: a post hoc analysis of two randomised controlled trials
- PMID: 36007980
- PMCID: PMC9422833
- DOI: 10.1136/bmjresp-2022-001271
Asthma control with ICS-formoterol reliever versus maintenance ICS and SABA reliever therapy: a post hoc analysis of two randomised controlled trials
Abstract
Background: In randomised controlled trials, as-needed inhaled corticosteroid (ICS)-formoterol reliever therapy reduces severe exacerbation risk compared with maintenance ICS plus short-acting beta2-agonist (SABA) reliever in adolescent and adult asthma, but results in slightly worse control of asthma symptoms, as measured by mean Asthma Control Questionnaire-5 (ACQ-5) score.
Objective: To assess the levels and changes in asthma control for as-needed budesonide-formoterol versus maintenance budesonide plus SABA in post hoc analyses from the Novel START and PRACTICAL clinical trials.
Methods: The number and proportion of participants at study end in each ACQ-5 category ('well-controlled', 'partly controlled' or 'inadequately controlled' symptoms), and in each responder category based on the minimal clinically important difference for ACQ-5 of 0.5 (improved, no change and worse) with as-needed budesonide-formoterol and maintenance budesonide plus SABA treatment were calculated.
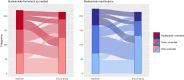
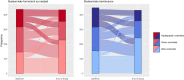
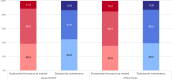
Results: With last observation carried forwards, 189/214 (88.3%) and 354/434 (81.6%) of patients in the budesonide-formoterol group had 'well-controlled' or 'partly controlled' symptoms at the end of the study, vs 183/214 (85.5%) and 358/431 (83.1%) in the budesonide maintenance group, for Novel START and PRACTICAL, respectively. The proportion of patients whose symptom control was either improved or unchanged from baseline was 190/214 (88.8%) and 368/434 (84.8%) for budesonide-formoterol, vs 185/214 (86.4%) and 376/431 (87.2%) for maintenance budesonide, in Novel START and PRACTICAL respectively.
Conclusions: There were no clinically important differences in the proportions of patients with 'well-controlled' or 'partly controlled' asthma symptoms, or proportions who improved or maintained their level of control, with as-needed budesonide-formoterol versus maintenance budesonide plus SABA.
Keywords: Asthma; Inhaler devices.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: LH, PB, CH, AE, RH, IB, MW, KO and MH have nothing to declare. IP reports speak fees from Aerocrine AB; speaker and consultant fees from Almirall and Novartis; speaker fees, payments for organisation of educational events, consultant fees, international scientific meeting sponsorship from AstraZeneca; speaker fees, consultant fees, international scientific meeting sponsorship from Boehringer Ingelheim; speaker fees, consultant fees, international scientific meeting sponsorship, research grant from Chiesi; speaker fees, payments for organisation of educational events, consultant fees, international scientific meeting sponsorship from GlaxoSmithKline, Regeneron Pharmaceuticals Inc, Sanofi, and Teva; consultant fees from Circassia, Dey Pharma, Genetech, Knopp Biosciences, Merk, MSD, RespiVert, and Schering-Plough; consultant fees and international scientific meeting sponsorship from Napp Pharmaceuticals. HKR reports support for the present manuscript from AstraZeneca (for Novel START) and the Health Research Council of New Zealand (for PRACTICAL); research grants from AstraZeneca, GlaxoSmithKline, Novartis; consulting fees from AstraZeneca and Novartis; independent medical education fees from AstraZeneca, GlaxoSmithKline, Teva, Boehringer-Ingelheim, Sanofi and Chiesi; participation on Advisory Boards for AstraZeneca, GlaxoSmithKline, Novartis, Chiesi and Sanofi; unpaid board roles for the Global Initiative for Asthma and the National Asthma Council (Australia). AP reports research grants from Chiesi, AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Teva, and Sanofi; consulting fees from Chiesi, AstraZeneca, GlaxoSmithKline, Novartis, Sanofi, IQVIA, Avillion, Elpen Pharmaceuticals, MSD; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events, from Chiesi, AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Menarini, Novartis, Zambon, Mundipharma, Teva, Sanofi, Edmond Pharma, IQIVA; Participation on a Data Safety Monitoring Board or Advisory Board for Chiesi, AstraZeneca, GlaxoSmithKline, Novartis, Sanofi, IQVIA, Avillion, Elpen Pharmaceuticals, MSD. RB reports support for the present manuscript from AstraZeneca (for Novel START) and the Health Research Council of New Zealand (for PRACTICAL); research grants from AstraZeneca, Genentech, the Health Research Council of New Zealand and Cure Kids NZ; fees and support from AstraZeneca, Cipla, Avillion, and the Asthma and Respiratory Foundation NZ for presentations, AdBoards, attending meetings and travel, participation on advisory boards.
Figures
Similar articles
-
[Budesonide/formoterol maintenance and reliever therapy. A new treatment approach for adult patients with asthma].Med Klin (Munich). 2008 May 15;103(5):299-310. doi: 10.1007/s00063-008-1050-y. Med Klin (Munich). 2008. PMID: 18484216 Review. German.
-
Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial.Lancet. 2019 Sep 14;394(10202):919-928. doi: 10.1016/S0140-6736(19)31948-8. Epub 2019 Aug 23. Lancet. 2019. PMID: 31451207 Clinical Trial.
-
The effect of budesonide/formoterol maintenance and reliever therapy on the risk of severe asthma exacerbations following episodes of high reliever use: an exploratory analysis of two randomised, controlled studies with comparisons to standard therapy.Respir Res. 2012 Jul 20;13(1):59. doi: 10.1186/1465-9921-13-59. Respir Res. 2012. PMID: 22816878 Free PMC article. Clinical Trial.
-
Evaluation of Budesonide-Formoterol for Maintenance and Reliever Therapy Among Patients With Poorly Controlled Asthma: A Systematic Review and Meta-analysis.JAMA Netw Open. 2022 Mar 1;5(3):e220615. doi: 10.1001/jamanetworkopen.2022.0615. JAMA Netw Open. 2022. PMID: 35230437 Free PMC article.
-
Budesonide/formoterol maintenance and reliever therapy: a new treatment approach for adult patients with asthma.Curr Med Res Opin. 2007 Aug;23(8):1867-78. doi: 10.1185/030079907X210769. Curr Med Res Opin. 2007. PMID: 17605896 Review.
Cited by
-
Effect of randomized treatment withdrawal of budesonide oral suspension on clinically relevant efficacy outcomes in patients with eosinophilic esophagitis: a post hoc analysis.Therap Adv Gastroenterol. 2024 Dec 23;17:17562848241307602. doi: 10.1177/17562848241307602. eCollection 2024. Therap Adv Gastroenterol. 2024. PMID: 39735351 Free PMC article.
-
Independent risk factors of asthma exacerbations: 3-year follow-up in a single-center prospective cohort study.Ann Transl Med. 2022 Dec;10(24):1353. doi: 10.21037/atm-22-5918. Ann Transl Med. 2022. PMID: 36660650 Free PMC article.
-
Can we measure whether asthma guidelines lead to improved care?NPJ Prim Care Respir Med. 2024 Jun 27;34(1):16. doi: 10.1038/s41533-024-00379-6. NPJ Prim Care Respir Med. 2024. PMID: 38937520 Free PMC article. Review.
References
-
- Global Initiative for Asthma (GINA) . Global strategy for asthma management and prevention, 2021. Available: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
