Indicators of central sensitization in chronic neuropathic pain after spinal cord injury
- PMID: 36008094
- PMCID: PMC9826442
- DOI: 10.1002/ejp.2028
Indicators of central sensitization in chronic neuropathic pain after spinal cord injury
Abstract
Background: Central sensitization is considered a key mechanism underlying neuropathic pain (NP) after spinal cord injury (SCI).
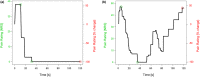
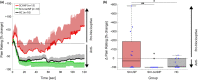
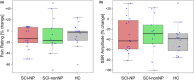
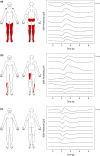
Methods: Two novel proxies for central sensitization were investigated in thoracic SCI subjects with (SCI-NP) and without NP (SCI-nonNP) compared to healthy controls (HC). Specifically, temporal summation of pain (TSP) was investigated by examining pain ratings during a 2-min tonic heat application to the volar forearm. Additionally, palmar heat-induced sympathetic skin responses (SSR) were recorded in order to reveal changes in pain-autonomic interaction above the lesion level. Pain extent was assessed as the percentage of the body area and the number of body regions being affected by NP.
Results: Enhanced TSP was observed in SCI-NP (+66%) compared to SCI-nonNP (-75%, p = 0.009) and HC (-59%, p = 0.021). In contrast, no group differences were found (p = 0.685) for SSR habituation. However, pain extent in SCI-NP was positively correlated with deficient SSR habituation (body area: r = 0.561, p = 0.024; body regions: r = 0.564, p = 0.023).
Conclusions: These results support the value of TSP and heat-induced SSRs as proxies for central sensitization in widespread neuropathic pain syndromes after SCI. Measures of pain-autonomic interaction emerged as a promising tool for the objective investigation of sensitized neuronal states in chronic pain conditions.
Significance: We present two surrogate readouts for central sensitization in neuropathic pain following SCI. On the one hand, temporal summation of tonic heat pain is enhanced in subjects with neuropathic pain. On the other hand, pain-autonomic interaction reveals potential advanced measures in chronic pain, as subjects with a high extent of neuropathic pain showed diminished habituation of pain-induced sympathetic measures. A possible implication for clinical practice is constituted by an improved assessment of neuronal hyperexcitability potentially enabling mechanism-based treatment.
© 2022 The Authors. European Journal of Pain published by John Wiley & Sons Ltd on behalf of European Pain Federation - EFIC ®.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding this work.
Figures

Similar articles
-
Anti- and Pro-Nociceptive mechanisms in neuropathic pain after human spinal cord injury.Eur J Pain. 2022 Nov;26(10):2176-2187. doi: 10.1002/ejp.2029. Epub 2022 Sep 10. Eur J Pain. 2022. PMID: 36000307 Free PMC article.
-
Deficient conditioned pain modulation after spinal cord injury correlates with clinical spontaneous pain measures.Pain. 2015 Feb;156(2):260-272. doi: 10.1097/01.j.pain.0000460306.48701.f9. Pain. 2015. PMID: 25599447
-
Identifying Discomplete Spinal Lesions: New Evidence from Pain-Autonomic Interaction in Spinal Cord Injury.J Neurotrauma. 2021 Dec;38(24):3456-3466. doi: 10.1089/neu.2021.0280. J Neurotrauma. 2021. PMID: 34806429
-
Regional Hyperexcitability and Chronic Neuropathic Pain Following Spinal Cord Injury.Cell Mol Neurobiol. 2020 Aug;40(6):861-878. doi: 10.1007/s10571-020-00785-7. Epub 2020 Jan 18. Cell Mol Neurobiol. 2020. PMID: 31955281 Free PMC article. Review.
-
Neuropathic Pain Induced by Spinal Cord Injury from the Glia Perspective and Its Treatment.Cell Mol Neurobiol. 2024 Nov 28;44(1):81. doi: 10.1007/s10571-024-01517-x. Cell Mol Neurobiol. 2024. PMID: 39607514 Free PMC article. Review.
Cited by
-
Central neuropathic pain.Nat Rev Dis Primers. 2023 Dec 21;9(1):73. doi: 10.1038/s41572-023-00484-9. Nat Rev Dis Primers. 2023. PMID: 38129427 Free PMC article. Review.
-
Habituation to Pain in Patients with Chronic Pain: Clinical Implications and Future Directions.J Clin Med. 2023 Jun 27;12(13):4305. doi: 10.3390/jcm12134305. J Clin Med. 2023. PMID: 37445339 Free PMC article. Review.
-
Priming of the autonomic nervous system after an experimental human pain model.J Neurophysiol. 2023 Aug 1;130(2):436-446. doi: 10.1152/jn.00064.2023. Epub 2023 Jul 5. J Neurophysiol. 2023. PMID: 37405990 Free PMC article.
-
Data-driven dynamic profiles of tonic heat pain perception in pain-free volunteers are associated with differences in anandamide levels.Sci Rep. 2024 Jul 26;14(1):17238. doi: 10.1038/s41598-024-67401-2. Sci Rep. 2024. PMID: 39060336 Free PMC article.
-
In vivo imaging of the neuronal response to spinal cord injury: a narrative review.Neural Regen Res. 2024 Apr;19(4):811-817. doi: 10.4103/1673-5374.382225. Neural Regen Res. 2024. PMID: 37843216 Free PMC article. Review.
References
-
- Albu, S. , Gomez‐Soriano, J. , Avila‐Martin, G. , & Taylor, J. (2015). Deficient conditioned pain modulation after spinal cord injury correlates with clinical spontaneous pain measures. Pain, 156, 260–272. - PubMed
-
- Arendt‐Nielsen, L. , Morlion, B. , Perrot, S. , Dahan, A. , Dickenson, A. , Kress, H. G. , Wells, C. , Bouhassira, D. , & Mohr, D. A. (2018). Assessment and manifestation of central sensitisation across different chronic pain conditions. European Journal of Pain, 22, 216–241. - PubMed
-
- Baron, R. , Binder, A. , & Wasner, G. (2010). Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurology, 9, 807–819. - PubMed
-
- Baron, R. , Hans, G. , & Dickenson, A. H. (2013). Peripheral input and its importance for central sensitization. Annals of Neurology, 74, 630–636. - PubMed
-
- Benarroch, E. E. (2006). Pain‐autonomic interactions. Neurological Sciences, 27(Suppl 2), S130–S133. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

