First-in-Humans Evaluation of Safety and Dosimetry of 64Cu-LLP2A for PET Imaging
- PMID: 36008121
- PMCID: PMC9902845
- DOI: 10.2967/jnumed.122.264349
First-in-Humans Evaluation of Safety and Dosimetry of 64Cu-LLP2A for PET Imaging
Abstract
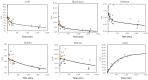
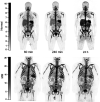
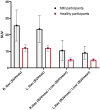
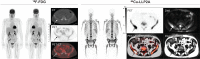
There remains an unmet need for molecularly targeted imaging agents for multiple myeloma (MM). The integrin very late antigen 4 (VLA4), is differentially expressed in malignant MM cells and in pathogenic inflammatory microenvironmental cells. [64Cu]Cu-CB-TE1A1P-LLP2A (64Cu-LLP2A) is a VLA4-targeted, high-affinity radiopharmaceutical with promising utility for managing patients diagnosed with MM. Here, we evaluated the safety and human radiation dosimetry of 64Cu-LLP2A for potential use in MM patients. Methods: A single-dose [natCu]Cu-LLP2A (Cu-LLP2A) tolerability and toxicity study was performed on CD-1 (Hsd:ICR) male and female mice. 64Cu-LLP2A was synthesized in accordance with good-manufacturing-practice-compliant procedures. Three MM patients and six healthy participants underwent 64Cu-LLP2A-PET/CT or PET/MRI at up to 3 time points to help determine tracer biodistribution, pharmacokinetics, and radiation dosimetry. Time-activity curves were plotted for each participant. Mean organ-absorbed doses and effective doses were calculated using the OLINDA software. Tracer bioactivity was evaluated via cell-binding assays, and metabolites from human blood samples were analyzed with analytic radio-high-performance liquid chromatography. When feasible, VLA4 expression was evaluated in the biopsy tissues using 14-color flow cytometry. Results: A 150-fold mass excess of the desired imaging dose was tolerated well in male and female CD-1 mice (no observed adverse effect level). Time-activity curves from human imaging data showed rapid tracer clearance from blood via the kidneys and bladder. The effective dose of 64Cu-LLP2A in humans was 0.036 ± 0.006 mSv/MBq, and the spleen had the highest organ uptake, 0.142 ± 0.034 mSv/MBq. Among all tissues, the red marrow demonstrated the highest residence time. Image quality analysis supports an early imaging time (4-5 h after injection of the radiotracer) as optimal. Cell studies showed statistically significant blocking for the tracer produced for all human studies (82.42% ± 13.47%). Blood metabolism studies confirmed a stable product peak (>90%) up to 1 h after injection of the radiopharmaceutical. No clinical or laboratory adverse events related to 64Cu-LLP2A were observed in the human participants. Conclusion: 64Cu-LLP2A exhibited a favorable dosimetry and safety profile for use in humans.
Keywords: dosimetry; first-in-humans; radiochemistry; radiopharmaceuticals; safety; translational imaging.
© 2023 by the Society of Nuclear Medicine and Molecular Imaging.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
