Hippocampal circuit dysfunction in psychosis
- PMID: 36008395
- PMCID: PMC9411597
- DOI: 10.1038/s41398-022-02115-5
Hippocampal circuit dysfunction in psychosis
Erratum in
-
Correction: Hippocampal circuit dysfunction in psychosis.Transl Psychiatry. 2022 Sep 12;12(1):382. doi: 10.1038/s41398-022-02154-y. Transl Psychiatry. 2022. PMID: 36096989 Free PMC article. No abstract available.
Abstract
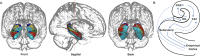
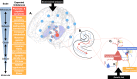
Despite strong evidence of the neurodevelopmental origins of psychosis, current pharmacological treatment is not usually initiated until after a clinical diagnosis is made, and is focussed on antagonising striatal dopamine receptors. These drugs are only partially effective, have serious side effects, fail to alleviate the negative and cognitive symptoms of the disorder, and are not useful as a preventive treatment. In recent years, attention has turned to upstream brain regions that regulate striatal dopamine function, such as the hippocampus. This review draws together these recent data to discuss why the hippocampus may be especially vulnerable in the pathophysiology of psychosis. First, we describe the neurodevelopmental trajectory of the hippocampus and its susceptibility to dysfunction, exploring this region's proneness to structural and functional imbalances, metabolic pressures, and oxidative stress. We then examine mechanisms of hippocampal dysfunction in psychosis and in individuals at high-risk for psychosis and discuss how and when hippocampal abnormalities may be targeted in these groups. We conclude with future directions for prospective studies to unlock the discovery of novel therapeutic strategies targeting hippocampal circuit imbalances to prevent or delay the onset of psychosis.
© 2022. The Author(s).
Conflict of interest statement
AAG has received consulting fees from Alkermes, Lundbeck, Takeda, Roche, Lyra, Concert, and SynAgile. RM has received honoraria from Janssen and Otsuka. The other authors declare no conflicts of interest.
Figures

References
-
- Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

