Tripterygium wilfordii cytochrome P450s catalyze the methyl shift and epoxidations in the biosynthesis of triptonide
- PMID: 36008399
- PMCID: PMC9411204
- DOI: 10.1038/s41467-022-32667-5
Tripterygium wilfordii cytochrome P450s catalyze the methyl shift and epoxidations in the biosynthesis of triptonide
Erratum in
-
Author Correction: Tripterygium wilfordii cytochrome P450s catalyze the methyl shift and epoxidations in the biosynthesis of triptonide.Nat Commun. 2025 Jul 24;16(1):6811. doi: 10.1038/s41467-025-62209-8. Nat Commun. 2025. PMID: 40707453 Free PMC article. No abstract available.
Abstract
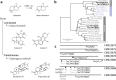
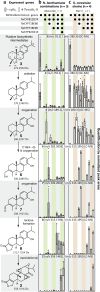
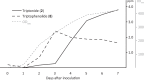
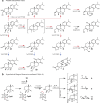
The diterpenoid triepoxides triptolide and triptonide from Tripterygium wilfordii (thunder god wine) exhibit unique bioactivities with potential uses in disease treatment and as a non-hormonal male contraceptives. Here, we show that cytochrome P450s (CYPs) from the CYP71BE subfamily catalyze an unprecedented 18(4→3) methyl shift required for biosynthesis of the abeo-abietane core structure present in diterpenoid triepoxides and in several other plant diterpenoids. In combination with two CYPs of the CYP82D subfamily, four CYPs from T. wilfordii are shown to constitute the minimal set of biosynthetic genes that enables triptonide biosynthesis using Nicotiana benthamiana and Saccharomyces cerevisiae as heterologous hosts. In addition, co-expression of a specific T. wilfordii cytochrome b5 (Twcytb5-A) increases triptonide output more than 9-fold in S. cerevisiae and affords isolation and structure elucidation by NMR spectroscopic analyses of 18 diterpenoids, providing insights into the biosynthesis of diterpenoid triepoxides. Our findings pave the way for diterpenoid triepoxide production via fermentation.
© 2022. The Author(s).
Conflict of interest statement
N.L.H., V.F., and J.A-R. are inventors of the patent entitled “Production of oxygenated diterpenoid compounds” (application number: PCT/EP2021/073656) related to the cytochrome P450 enzymes described in the paper. J.A-R. has established TriptoBIO to commercialize the patented technology. Other authors claim no competing interests.
Figures
References
-
- Kupchan, S. M., Court, W. A., Dailey, R. G., Gilmore, C. J. & Bryan, R. F. Tumor inhibitors. LXXIV. Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J. Am. Chem. Soc.94, 7194–7195 (1972). - PubMed
-
- Dyer, C. A. et al. Accelerated follicle depletion in vitro and in vivo in sprague-dawley rats using the combination of 4-vinylcyclohexene diepoxide and triptolide. J. Zoo. Wildl. Med.44, S9–S17 (2013). - PubMed
-
- Kutney, J. P. et al. Cultivation of Tripterygium wilfordii tissue cultures for the production of the cytotoxic diterpene tripdiolide. Planta Med48, 158–163 (1983). - PubMed
-
- Kutney, J. P. et al. Cytotoxic diterpenes triptolide, tripdiolide, and cytotoxic triterpenes from tissue cultures of Tripterygium wilfordii. Can. J. Chem.59, 2677–2683 (1981).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

