Designed peptides as nanomolar cross-amyloid inhibitors acting via supramolecular nanofiber co-assembly
- PMID: 36008417
- PMCID: PMC9411207
- DOI: 10.1038/s41467-022-32688-0
Designed peptides as nanomolar cross-amyloid inhibitors acting via supramolecular nanofiber co-assembly
Abstract
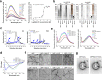
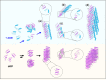
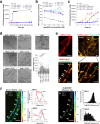
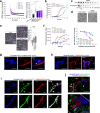
Amyloid self-assembly is linked to numerous devastating cell-degenerative diseases. However, designing inhibitors of this pathogenic process remains a major challenge. Cross-interactions between amyloid-β peptide (Aβ) and islet amyloid polypeptide (IAPP), key polypeptides of Alzheimer's disease (AD) and type 2 diabetes (T2D), have been suggested to link AD with T2D pathogenesis. Here, we show that constrained peptides designed to mimic the Aβ amyloid core (ACMs) are nanomolar cross-amyloid inhibitors of both IAPP and Aβ42 and effectively suppress reciprocal cross-seeding. Remarkably, ACMs act by co-assembling with IAPP or Aβ42 into amyloid fibril-resembling but non-toxic nanofibers and their highly ordered superstructures. Co-assembled nanofibers exhibit various potentially beneficial features including thermolability, proteolytic degradability, and effective cellular clearance which are reminiscent of labile/reversible functional amyloids. ACMs are thus promising leads for potent anti-amyloid drugs in both T2D and AD while the supramolecular nanofiber co-assemblies should inform the design of novel functional (hetero-)amyloid-based nanomaterials for biomedical/biotechnological applications.
© 2022. The Author(s).
Conflict of interest statement
A.K., J.B., and K.T. are co-inventors of the European patent application 22 158 021.0 (applicant Technical University of Munich) (status: pending) related to ACMs, their hetero-assemblies, and potential biomedical applications. The remaining authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

