Hydrogen peroxide initiates oxidative stress and proteomic alterations in meningothelial cells
- PMID: 36008468
- PMCID: PMC9411503
- DOI: 10.1038/s41598-022-18548-3
Hydrogen peroxide initiates oxidative stress and proteomic alterations in meningothelial cells
Abstract
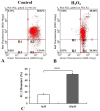
Meningothelial cells (MECs) are fundamental cells of the sheaths covering the brain and optic nerve, where they build a brain/optic nerve-cerebral spinal fluid (CSF) barrier that prevents the free flow of CSF from the subarachnoid space, but their exact roles and underlying mechanisms remain unclear. Our attempt here was to investigate the influence elicited by hydrogen peroxide (H2O2) on functional changes of MECs. Our study showed that cell viability of MECs was inhibited after cells were exposed to oxidative agents. Cells subjected to H2O2 at the concentration of 150 µM for 24 h and 48 h exhibited an elevation of reactive oxygen species (ROS) activity, decrease of total antioxidant capacity (T-AOC) level and reduced mitochondrial membrane potential (ΔΨm) compared with control cells. 95 protein spots with more than twofold difference were detected in two dimensional electrophoresis (2DE) gels through proteomics assay following H2O2 exposure for 48 h, 10 proteins were identified through TOF/MS analysis. Among the proteomic changes explored, 8 proteins related to energy metabolism, mitochondrial function, structural regulation, and cell cycle control were downregulated. Our study provides key insights that enhance our understanding of the role of MECs in the pathology of brain and optic nerve disorders.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Preincubation with a low-dose hydrogen peroxide enhances anti-oxidative stress ability of BMSCs.J Orthop Surg Res. 2020 Sep 9;15(1):392. doi: 10.1186/s13018-020-01916-y. J Orthop Surg Res. 2020. PMID: 32907609 Free PMC article.
-
Punicalagin reduces H2O2-induced cytotoxicity and apoptosis in PC12 cells by modulating the levels of reactive oxygen species.Nutr Neurosci. 2018 Jul;21(6):447-454. doi: 10.1080/1028415X.2017.1306935. Epub 2017 Apr 9. Nutr Neurosci. 2018. PMID: 28393656
-
Carboxymethylated chitosan protects Schwann cells against hydrogen peroxide-induced apoptosis by inhibiting oxidative stress and mitochondria dependent pathway.Eur J Pharmacol. 2018 Apr 15;825:48-56. doi: 10.1016/j.ejphar.2018.02.024. Epub 2018 Feb 17. Eur J Pharmacol. 2018. PMID: 29462593
-
Apoptosis in resistance arteries induced by hydrogen peroxide: greater resilience of endothelium versus smooth muscle.Am J Physiol Heart Circ Physiol. 2021 Apr 1;320(4):H1625-H1633. doi: 10.1152/ajpheart.00956.2020. Epub 2021 Feb 19. Am J Physiol Heart Circ Physiol. 2021. PMID: 33606587 Free PMC article. Review.
-
A radical shift in perspective: mitochondria as regulators of reactive oxygen species.J Exp Biol. 2017 Apr 1;220(Pt 7):1170-1180. doi: 10.1242/jeb.132142. J Exp Biol. 2017. PMID: 28356365 Review.
Cited by
-
Lauric acid with or without levodopa ameliorates Parkinsonism in genetically modified model of Drosophila melanogaster via the oxidative-inflammatory-apoptotic pathway.Brain Behav. 2024 Sep;14(9):e70001. doi: 10.1002/brb3.70001. Brain Behav. 2024. PMID: 39245995 Free PMC article.
-
Chronic unpredictable stress induces anxiety-like behavior and oxidative stress, leading to diminished ovarian reserve.Sci Rep. 2024 Dec 28;14(1):30681. doi: 10.1038/s41598-024-76717-y. Sci Rep. 2024. PMID: 39730417 Free PMC article.
-
Oxidative stress induced by hydrogen peroxide disrupts zebrafish visual development by altering apoptosis, antioxidant and estrogen related genes.Sci Rep. 2024 Jun 24;14(1):14454. doi: 10.1038/s41598-024-64933-5. Sci Rep. 2024. PMID: 38914633 Free PMC article.
-
Fucoxanthin diminishes oxidative stress damage in human placenta-derived mesenchymal stem cells through the PI3K/Akt/Nrf-2 pathway.Sci Rep. 2023 Dec 27;13(1):22974. doi: 10.1038/s41598-023-49751-5. Sci Rep. 2023. PMID: 38151503 Free PMC article.
-
Amyloid beta-induced signalling in leptomeningeal cells and its impact on astrocyte response.Mol Cell Biochem. 2025 Apr;480(4):2645-2660. doi: 10.1007/s11010-024-05151-5. Epub 2024 Nov 5. Mol Cell Biochem. 2025. PMID: 39499391
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

