Weak catch bonds make strong networks
- PMID: 36008604
- PMCID: PMC7613626
- DOI: 10.1038/s41563-022-01288-0
Weak catch bonds make strong networks
Abstract
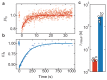
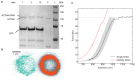
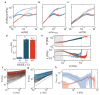
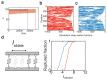
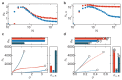
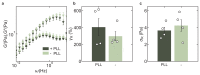
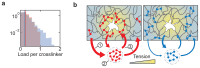
Molecular catch bonds are ubiquitous in biology and essential for processes like leucocyte extravasion1 and cellular mechanosensing2. Unlike normal (slip) bonds, catch bonds strengthen under tension. The current paradigm is that this feature provides 'strength on demand3', thus enabling cells to increase rigidity under stress1,4-6. However, catch bonds are often weaker than slip bonds because they have cryptic binding sites that are usually buried7,8. Here we show that catch bonds render reconstituted cytoskeletal actin networks stronger than slip bonds, even though the individual bonds are weaker. Simulations show that slip bonds remain trapped in stress-free areas, whereas weak binding allows catch bonds to mitigate crack initiation by moving to high-tension areas. This 'dissociation on demand' explains how cells combine mechanical strength with the adaptability required for shape change, and is relevant to diseases where catch bonding is compromised7,9, including focal segmental glomerulosclerosis10 caused by the α-actinin-4 mutant studied here. We surmise that catch bonds are the key to create life-like materials.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures







References
-
- Marshall BT, et al. Direct observation of catch bonds involving cell-adhesion molecules. Nature. 2003;423:190–3. - PubMed
-
- Mbanga BL, Iyer BVS, Yashin VV, Balazs AC. Tuning the Mechanical Properties of Polymer-Grafted Nanoparticle Networks through the Use of Biomimetic Catch Bonds. Macromolecules. 2016;49:1353–1361.
-
- Thomas WE, Trintchina E, Forero M, Vogel V, Sokurenko EV. Bacterial Adhesion to Target Cells Enhanced by Shear Force. Cell. 2002;109:913–923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

