Repeated intravenous administration of hiPSC-MSCs enhance the efficacy of cell-based therapy in tissue regeneration
- PMID: 36008710
- PMCID: PMC9411616
- DOI: 10.1038/s42003-022-03833-8
Repeated intravenous administration of hiPSC-MSCs enhance the efficacy of cell-based therapy in tissue regeneration
Abstract
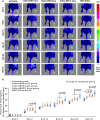
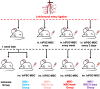
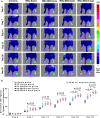
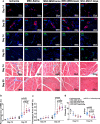
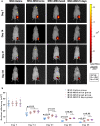
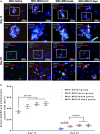
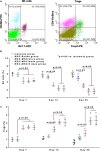
We seek to demonstrate whether therapeutic efficacy can be improved by combination of repeated intravenous administration and local transplantation of human induced pluripotential stem cell derived MSCs (hiPSC-MSCs). In this study, mice model of hind-limb ischemia is established by ligation of left femoral artery. hiPSC-MSCs (5 × 105) is intravenously administrated immediately after induction of hind limb ischemia with or without following intravenous administration of hiPSC-MSCs every week or every 3 days. Intramuscular transplantation of hiPSC-MSCs (3 × 106) is performed one week after induction of hind-limb ischemia. We compare the therapeutic efficacy and cell survival of intramuscular transplantation of hiPSC-MSCs with or without a single or repeated intravenous administration of hiPSC-MSCs. Repeated intravenous administration of hiPSC-MSCs can increase splenic regulatory T cells (Tregs) activation, decrease splenic natural killer (NK) cells expression, promote the polarization of M2 macrophages in the ischemic area and improved blood perfusion in the ischemic limbs. The improved therapeutic efficacy of MSC-based therapy is due to both increased engraftment of intramuscular transplanted hiPSC-MSCs and intravenous infused hiPSC-MSCs. In conclusion, our study support a combination of repeated systemic infusion and local transplantation of hiPSC-MSCs for cardiovascular disease.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Madonna R, et al. Position Paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: Cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur. Heart J. 2016;37:1789–1798. doi: 10.1093/eurheartj/ehw113. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

