A case study of combined neoadjuvant chemotherapy and neoadjuvant immunotherapy in resectable locally advanced esophageal cancer
- PMID: 36008813
- PMCID: PMC9414113
- DOI: 10.1186/s12957-022-02732-w
A case study of combined neoadjuvant chemotherapy and neoadjuvant immunotherapy in resectable locally advanced esophageal cancer
Abstract
Background: The prognosis of patients under existing neoadjuvant chemotherapy or neoadjuvant chemoradiotherapy requires improvement. Whereas programmed cell death 1 (PD-1) inhibitors have shown promising response in advanced esophageal cancer, they have not been used in the perioperative treatment of resectable locally advanced esophageal cancer. Whether immunotherapy can be incorporated into neoadjuvant therapy has became a challenging question for researchers.
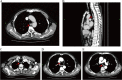
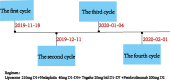
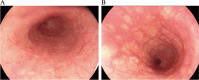
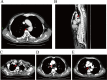
Case presentation: We present a case of a 65-year-old male who had a history of progressive dysphagia for approximately 1 month. He underwent pertinent studies including computed tomography (CT),gastroscopy,and pathological biopsy resulting in a diagnosis of medium-low differentiated squamous carcinoma of the thoracic segment of the esophagus (cT2N2M0 stage III). After 4 cycles of neoadjuvant chemotherapy combined with immunotherapy, gastroscopy showed the lesion in the esophagus was no longer present. Subsequently, the patient received thoracoscopic radical resection of esophageal cancer and achieved a pathological complete response (pCR) in postoperative pathological evaluation. During the whole treatment, no adverse effect was recorded and to date no evidence of recurrence has been recorded.
Conclusion: Our report suggest that neoadjuvant chemotherapy combined with immunotherapy not only improve the R0 resection and pCR rate in patients with resectable locally advanced esophageal cancer, but also the adverse effects are within the control range. However, the selection of therapeutic strategy, predictors of response to treatment, and interval time between neoadjuvant treatment and surgery still await more reliable evidence-based studies with large prospective samples.
Keywords: Esophageal cancer; Immunotherapy; Neoadjuvant therapy; PD-1 inhibitor.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Perioperative tislelizumab plus chemotherapy for locally advanced resectable thoracic esophageal squamous cell carcinoma trial: a prospective single-arm, phase II study (PILOT trial).BMC Cancer. 2023 Dec 15;23(1):1237. doi: 10.1186/s12885-023-11747-9. BMC Cancer. 2023. PMID: 38102553 Free PMC article. Clinical Trial.
-
Neoadjuvant PD-1 blockade combined with chemotherapy is not superior to neoadjuvant chemotherapy alone in resectable locally advanced esophageal carcinoma.World J Surg Oncol. 2023 Feb 3;21(1):33. doi: 10.1186/s12957-023-02915-z. World J Surg Oncol. 2023. PMID: 36737768 Free PMC article.
-
Case report: A case study of neoadjuvant immunochemotherapy for locally advanced esophageal squamous carcinoma.Front Oncol. 2024 Jul 4;14:1332314. doi: 10.3389/fonc.2024.1332314. eCollection 2024. Front Oncol. 2024. PMID: 39026974 Free PMC article.
-
Comparison of neoadjuvant immunotherapy versus routine neoadjuvant therapy for patients with locally advanced esophageal cancer: A systematic review and meta-analysis.Front Immunol. 2023 Mar 23;14:1108213. doi: 10.3389/fimmu.2023.1108213. eCollection 2023. Front Immunol. 2023. PMID: 37033991 Free PMC article.
-
Evaluation of Clinical and Safety Outcomes of Neoadjuvant Immunotherapy Combined With Chemotherapy for Patients With Resectable Esophageal Cancer: A Systematic Review and Meta-analysis.JAMA Netw Open. 2022 Nov 1;5(11):e2239778. doi: 10.1001/jamanetworkopen.2022.39778. JAMA Netw Open. 2022. PMID: 36322089 Free PMC article.
References
-
- Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–1517. doi: 10.1016/S1470-2045(19)30626-6. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

